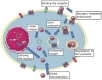
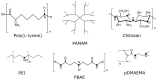
Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications
- PMID: 37109432
- PMCID: PMC10142071
- DOI: 10.3390/life13040903
Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications
Abstract
Over the past decades, non-viral DNA and RNA delivery systems have been intensively studied as an alternative to viral vectors. Despite the most significant advantage over viruses, such as the lack of immunogenicity and cytotoxicity, the widespread use of non-viral carriers in clinical practice is still limited due to the insufficient efficacy associated with the difficulties of overcoming extracellular and intracellular barriers. Overcoming barriers by non-viral carriers is facilitated by their chemical structure, surface charge, as well as developed modifications. Currently, there are many different forms of non-viral carriers for various applications. This review aimed to summarize recent developments based on the essential requirements for non-viral carriers for gene therapy.
Keywords: endocytosis; exosomes; gene delivery; liposomes; microvesicles; nanomaterials; non-viral carriers; peptide-based carriers; polyplexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers.Front Pharmacol. 2018 Aug 21;9:971. doi: 10.3389/fphar.2018.00971. eCollection 2018. Front Pharmacol. 2018. PMID: 30186185 Free PMC article. Review.
-
Biomedical applications of nanomaterials in the advancement of nucleic acid therapy: Mechanistic challenges, delivery strategies, and therapeutic applications.Int J Biol Macromol. 2023 Jun 30;241:124582. doi: 10.1016/j.ijbiomac.2023.124582. Epub 2023 Apr 26. Int J Biol Macromol. 2023. PMID: 37116843 Review.
-
Peptide modules for overcoming barriers of nucleic acids transport to cells.Curr Top Med Chem. 2016;16(3):330-42. doi: 10.2174/1568026615666150812120755. Curr Top Med Chem. 2016. PMID: 26265355 Review.
-
Advances in modification and delivery of nucleic acid drugs.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Aug 25;52(4):417-428. doi: 10.3724/zdxbyxb-2023-0130. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37643976 Free PMC article. Review. Chinese, English.
-
Clinical applications for exosomes: Are we there yet?Br J Pharmacol. 2021 Jun;178(12):2375-2392. doi: 10.1111/bph.15432. Epub 2021 May 3. Br J Pharmacol. 2021. PMID: 33751579 Free PMC article. Review.
Cited by
-
Gene therapy for retinal diseases: From genetics to treatment.Indian J Ophthalmol. 2024 Aug 1;72(8):1091-1101. doi: 10.4103/IJO.IJO_2902_23. Epub 2024 Jul 29. Indian J Ophthalmol. 2024. PMID: 39078952 Free PMC article. Review.
-
Serum-Resistant Ternary DNA Polyplexes for Suicide Gene Therapy of Uterine Leiomyoma.Int J Mol Sci. 2023 Dec 19;25(1):34. doi: 10.3390/ijms25010034. Int J Mol Sci. 2023. PMID: 38203202 Free PMC article.
-
Biomaterials-mediated CRISPR/Cas9 delivery: recent challenges and opportunities in gene therapy.Front Chem. 2023 Sep 28;11:1259435. doi: 10.3389/fchem.2023.1259435. eCollection 2023. Front Chem. 2023. PMID: 37841202 Free PMC article. Review.
-
Advancing Breast Cancer Therapeutics: Targeted Gene Delivery Systems Unveiling the Potential of Estrogen Receptor-Targeting Ligands.Biomater Res. 2024 Sep 24;28:0087. doi: 10.34133/bmr.0087. eCollection 2024. Biomater Res. 2024. PMID: 39319107 Free PMC article.
-
Nanotechnology-based non-viral vectors for gene delivery in cardiovascular diseases.Front Bioeng Biotechnol. 2024 Jan 18;12:1349077. doi: 10.3389/fbioe.2024.1349077. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38303912 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous