The Use of Hydrogels for the Treatment of Bone Osteosarcoma via Localized Drug-Delivery and Tissue Regeneration: A Narrative Review
- PMID: 37102886
- PMCID: PMC10137556
- DOI: 10.3390/gels9040274
The Use of Hydrogels for the Treatment of Bone Osteosarcoma via Localized Drug-Delivery and Tissue Regeneration: A Narrative Review
Abstract
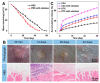
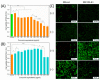
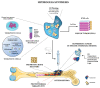
Osteosarcoma is a malignant tumor of bone that leads to poor mortality and morbidity. Management of this cancer through conventional methods involves invasive treatment options that place patients at an increased risk of adverse events. The use of hydrogels to target osteosarcoma has shown promising results both in vitro and in vivo to eradicate tumor cells while promoting bone regeneration. The loading of hydrogels with chemotherapeutic drugs provides a route for site-specific targeted therapy for osteosarcoma. Current studies demonstrate tumor regression in vivo and lysis of tumor cells in vitro when exposed to doped hydrogel scaffolds. Additionally, novel stimuli-responsive hydrogels are able to react with the tissue microenvironment to facilitate the controlled release of anti-tumor drugs and with biomechanical properties that can be modulated. This narrative review of the current literature discusses both in vitro and in vivo studies of different hydrogels, including stimuli-responsive, designed to treat bone osteosarcoma. Future applications to address patient treatment for this bone cancer are also discussed.
Keywords: bone; drug-delivery; hydrogels; osteosarcoma; scaffold; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In situ Co-Delivery of Doxorubicin and Cisplatin by Injectable Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment.Int J Nanomedicine. 2022 Mar 22;17:1309-1322. doi: 10.2147/IJN.S356453. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35345787 Free PMC article.
-
Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-co-Thermal Therapy and Bone Reconstruction.ACS Appl Mater Interfaces. 2021 Jul 14;13(27):31542-31553. doi: 10.1021/acsami.1c08775. Epub 2021 Jun 30. ACS Appl Mater Interfaces. 2021. PMID: 34191477
-
Sustained and Targeted Delivery of Self-Assembled Doxorubicin Nonapeptides Using pH-Responsive Hydrogels for Osteosarcoma Chemotherapy.Pharmaceutics. 2023 Feb 16;15(2):668. doi: 10.3390/pharmaceutics15020668. Pharmaceutics. 2023. PMID: 36839990 Free PMC article.
-
Designing Hydrogels for On-Demand Therapy.Acc Chem Res. 2017 Apr 18;50(4):669-679. doi: 10.1021/acs.accounts.6b00536. Epub 2017 Mar 16. Acc Chem Res. 2017. PMID: 28301139 Free PMC article. Review.
-
Bone scaffolds-based localized drugs delivery for osteosarcoma: current status and future perspective.Drug Deliv. 2024 Dec;31(1):2391001. doi: 10.1080/10717544.2024.2391001. Epub 2024 Sep 6. Drug Deliv. 2024. PMID: 39239763 Free PMC article. Review.
Cited by
-
Mesenchymal Stem/Stromal Cells: Immunomodulatory and Bone Regeneration Potential after Tumor Excision in Osteosarcoma Patients.Bioengineering (Basel). 2023 Oct 13;10(10):1187. doi: 10.3390/bioengineering10101187. Bioengineering (Basel). 2023. PMID: 37892917 Free PMC article. Review.
-
Use of Hydrogels in Regenerative Medicine: Focus on Mechanical Properties.Int J Mol Sci. 2024 Oct 24;25(21):11426. doi: 10.3390/ijms252111426. Int J Mol Sci. 2024. PMID: 39518979 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

