Mycobacterium tuberculosis and SARS-CoV-2 co-infections: The knowns and unknowns
- PMID: 37091987
- PMCID: PMC10082467
- DOI: 10.1016/j.isci.2023.106629
Mycobacterium tuberculosis and SARS-CoV-2 co-infections: The knowns and unknowns
Abstract
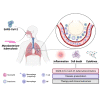
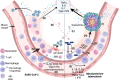
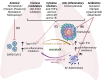
Health impacts of Mycobacterium tuberculosis (Mtb) and SARS-CoV-2 co-infections are not fully understood. Both pathogens modulate host responses and induce immunopathology with extensive lung damage. With a quarter of the world's population harboring latent TB, exploring the relationship between SARS-CoV-2 infection and its effect on the transition of Mtb from latent to active form is paramount to control this pathogen. The effects of active Mtb infection on establishment and severity of COVID-19 are also unknown, despite the ability of TB to orchestrate profound long-lasting immunopathologies in the lungs. Absence of mechanistic studies and co-infection models hinder the development of effective interventions to reduce the health impacts of SARS-CoV-2 and Mtb co-infection. Here, we highlight dysregulated immune responses induced by SARS-CoV-2 and Mtb, their potential interplay, and implications for co-infection in the lungs. As both pathogens master immunomodulation, we discuss relevant converging and diverging immune-related pathways underlying SARS-CoV-2 and Mtb co-infections.
Keywords: Bacteriology; Microbiology; Virology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Immune interaction between SARS-CoV-2 and Mycobacterium tuberculosis.Front Immunol. 2023 Sep 27;14:1254206. doi: 10.3389/fimmu.2023.1254206. eCollection 2023. Front Immunol. 2023. PMID: 37841282 Free PMC article. Review.
-
TB and COVID-19: An Exploration of the Characteristics and Resulting Complications of Co-infection.Front Biosci (Schol Ed). 2022 Mar 1;14(1):6. doi: 10.31083/j.fbs1401006. Front Biosci (Schol Ed). 2022. PMID: 35320917 Free PMC article. Review.
-
Tuberculosis.In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
-
Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-COV-2: similarities and differences.Front Immunol. 2023 Aug 17;14:1244556. doi: 10.3389/fimmu.2023.1244556. eCollection 2023. Front Immunol. 2023. PMID: 37662901 Free PMC article. Review.
-
Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis.Front Immunol. 2014 Apr 22;5:180. doi: 10.3389/fimmu.2014.00180. eCollection 2014. Front Immunol. 2014. PMID: 24795723 Free PMC article. Review.
Cited by
-
T Cell Response in Tuberculosis-Infected Patients Vaccinated against COVID-19.Microorganisms. 2023 Nov 19;11(11):2810. doi: 10.3390/microorganisms11112810. Microorganisms. 2023. PMID: 38004820 Free PMC article.
-
Co-infection of mice with SARS-CoV-2 and Mycobacterium tuberculosis limits early viral replication but does not affect mycobacterial loads.Front Immunol. 2023 Sep 1;14:1240419. doi: 10.3389/fimmu.2023.1240419. eCollection 2023. Front Immunol. 2023. PMID: 37720210 Free PMC article.
-
Animal models for COVID-19 and tuberculosis.Front Immunol. 2023 Aug 11;14:1223260. doi: 10.3389/fimmu.2023.1223260. eCollection 2023. Front Immunol. 2023. PMID: 37638020 Free PMC article. Review.
References
-
- World Health Organization . Annual Global TB Report of WHO; 2022. Annual Report of Tuberculosis. 1–68.
-
- Mhimbira F., Hiza H., Mbuba E., Hella J., Kamwela L., Sasamalo M., Ticlla M., Said K., Mhalu G., Chiryamkubi M., et al. Prevalence and clinical significance of respiratory viruses and bacteria detected in tuberculosis patients compared to household contact controls in Tanzania: a cohort study. Clin. Microbiol. Infection. 2019;25:107–107.e7. doi: 10.1016/j.cmi.2018.03.019. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

