Human Infrapatellar Fat Pad Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Fibroblast Proliferation by Regulating MT2A to Reduce Knee Arthrofibrosis
- PMID: 37091533
- PMCID: PMC10115539
- DOI: 10.1155/2023/9067621
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Fibroblast Proliferation by Regulating MT2A to Reduce Knee Arthrofibrosis
Abstract
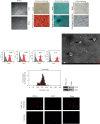
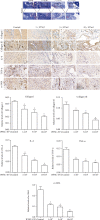
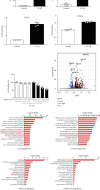
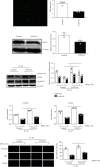
Knee arthrofibrosis is one of the most serious complications of knee surgery; however, its pathogenesis is unclear, and current treatment methods have not achieved satisfactory results. Mesenchymal stem cells (MSCs) have good anti-inflammatory and antifibrotic properties, and studies have reported that human infrapatellar fat pad-derived MSCs (IPFSCs) have the advantages of strong proliferative and differentiating ability, ease of acquisition, and minimal harm to the donor. Increasing evidence has shown that MSCs function through their paracrine extracellular vesicles (EVs). Our study is aimed at exploring the effects of human IPFSC-derived EVs (IPFSC-EVs) on knee arthrofibrosis and the underlying mechanisms in vivo and in vitro. In the in vivo study, injecting IPFSC-EVs into the knee joint cavity effectively reduced surgery-induced knee arthrofibrosis in rats. In the in vitro study, IPFSC-EVs were found to inhibit the proliferation of fibroblasts in the inflammatory environment. Additionally, we screened a potential IPFSC-EV molecular target, metallothionein 2A (MT2A), using RNA sequencing. We found that silencing MT2A partially reversed the inhibitory effect of IPFSC-EVs on fibroblast proliferation in the inflammatory environment. In conclusion, IPFSC-EVs inhibit the progression of knee arthrofibrosis by regulating MT2A, which inhibits fibroblast proliferation in the inflammatory environment.
Copyright © 2023 Dazhou Jia et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats.Stem Cells Transl Med. 2021 Mar;10(3):374-384. doi: 10.1002/sctm.20-0301. Epub 2020 Dec 15. Stem Cells Transl Med. 2021. PMID: 33319929 Free PMC article.
-
Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells.Int J Mol Sci. 2021 Mar 12;22(6):2889. doi: 10.3390/ijms22062889. Int J Mol Sci. 2021. PMID: 33809214 Free PMC article.
-
Therapeutic Effects of Hypoxic and Pro-Inflammatory Priming of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Inflammatory Arthritis.Int J Mol Sci. 2021 Dec 23;23(1):126. doi: 10.3390/ijms23010126. Int J Mol Sci. 2021. PMID: 35008555 Free PMC article.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
-
The Role of Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles (MSC-EVs) in Normal and Abnormal Hematopoiesis and Their Therapeutic Potential.J Clin Med. 2020 Mar 20;9(3):856. doi: 10.3390/jcm9030856. J Clin Med. 2020. PMID: 32245055 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

