Omics data integration facilitates target selection for new antiparasitic drugs against TriTryp infections
- PMID: 37089958
- PMCID: PMC10115950
- DOI: 10.3389/fphar.2023.1136321
Omics data integration facilitates target selection for new antiparasitic drugs against TriTryp infections
Abstract
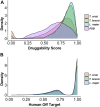
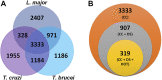
Introduction: Trypanosoma cruzi, Trypanosoma brucei, and Leishmania spp., commonly referred to as TriTryps, are a group of protozoan parasites that cause important human diseases affecting millions of people belonging to the most vulnerable populations worldwide. Current treatments have limited efficiencies and can cause serious side effects, so there is an urgent need to develop new control strategies. Presently, the identification and prioritization of appropriate targets can be aided by integrative genomic and computational approaches. Methods: In this work, we conducted a genome-wide multidimensional data integration strategy to prioritize drug targets. We included genomic, transcriptomic, metabolic, and protein structural data sources, to delineate candidate proteins with relevant features for target selection in drug development. Results and Discussion: Our final ranked list includes proteins shared by TriTryps and covers a range of biological functions including essential proteins for parasite survival or growth, oxidative stress-related enzymes, virulence factors, and proteins that are exclusive to these parasites. Our strategy found previously described candidates, which validates our approach as well as new proteins that can be attractive targets to consider during the initial steps of drug discovery.
Keywords: drug discovery; genomics; neglected disease; target selection; trypanosomatids.
Copyright © 2023 Rivara-Espasandín, Palumbo, Sosa, Radío, Turjanski, Sotelo-Silveira, Fernandez Do Porto and Smircich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Searching the Tritryp genomes for drug targets.Adv Exp Med Biol. 2008;625:133-40. doi: 10.1007/978-0-387-77570-8_11. Adv Exp Med Biol. 2008. PMID: 18365664 Free PMC article. Review.
-
State-of-the-art CRISPR/Cas9 Technology for Genome Editing in Trypanosomatids.J Eukaryot Microbiol. 2019 Nov;66(6):981-991. doi: 10.1111/jeu.12747. Epub 2019 Jul 7. J Eukaryot Microbiol. 2019. PMID: 31211904 Free PMC article. Review.
-
Rapid, Selection-Free, High-Efficiency Genome Editing in Protozoan Parasites Using CRISPR-Cas9 Ribonucleoproteins.mBio. 2017 Nov 7;8(6):e01788-17. doi: 10.1128/mBio.01788-17. mBio. 2017. PMID: 29114029 Free PMC article.
-
Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis.Int J Parasitol Drugs Drug Resist. 2018 Dec;8(3):430-439. doi: 10.1016/j.ijpddr.2018.09.006. Epub 2018 Sep 28. Int J Parasitol Drugs Drug Resist. 2018. PMID: 30293058 Free PMC article. Review.
-
Heme metabolism as a therapeutic target against protozoan parasites.J Drug Target. 2019 Aug;27(7):767-779. doi: 10.1080/1061186X.2018.1536982. Epub 2018 Oct 31. J Drug Target. 2019. PMID: 30332897 Review.
Cited by
-
Metallomics: An Essential Tool for the Study of Potential Antiparasitic Metallodrugs.ACS Omega. 2024 Mar 25;9(14):15744-15752. doi: 10.1021/acsomega.3c10200. eCollection 2024 Apr 9. ACS Omega. 2024. PMID: 38617611 Free PMC article. Review.
References
-
- Aronson N., Herwaldt B. L., Libman M., Pearson R., Lopez-Velez R., Weina P., et al. (2017). Diagnosis and treatment of leishmaniasis: Clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 96, 24–45. 10.4269/ajtmh.16-84256 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

