βγ G-proteins, but not regulators of G-protein signaling 4, modulate opioid-induced respiratory rate depression
- PMID: 37089428
- PMCID: PMC10117644
- DOI: 10.3389/fphys.2023.1043581
βγ G-proteins, but not regulators of G-protein signaling 4, modulate opioid-induced respiratory rate depression
Abstract
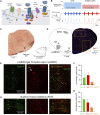
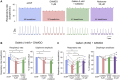
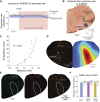
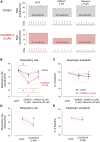
Opioid medications are the mainstay of pain management but present substantial side-effects such as respiratory depression which can be lethal with overdose. Most opioid drugs, such as fentanyl, act on opioid receptors such as the G-protein-coupled µ-opioid receptors (MOR). G-protein-coupled receptors activate pertussis toxin-sensitive G-proteins to inhibit neuronal activity. Binding of opioid ligands to MOR and subsequent activation G proteins βγ is modulated by regulator of G-protein signaling (RGS). The roles of G-proteins βγ and RGS in MOR-mediated inhibition of the respiratory network are not known. Using rodent models to pharmacologically modulate G-protein signaling, we aim to determine the roles of βγ G-proteins and RGS4. We showed that inhibition of βγ G-proteins using gallein perfused in the brainstem circuits regulating respiratory depression by opioid drugs results in complete reversal of respiratory depression. Blocking of RGS4 using CCG55014 did not change the respiratory depression induced by MOR activation despite co-expression of RGS4 and MORs in the brainstem. Our results suggest that neuronal inhibition by opioid drugs is mediated by G-proteins, but not by RGS4, which supports the concept that βγ G-proteins could be molecular targets to develop opioid overdose antidotes without the risks of re-narcotization often found with highly potent opioid drugs. On the other hand, RGS4 mediates opioid analgesia, but not respiratory depression, and RGS4 may be molecular targets to develop pain therapies without respiratory liability.
Keywords: regulators of G-protein-signaling; G-protein; breathing; medulla; opioid receptors; opioid-induced respiratory depression; preBotzinger complex.
Copyright © 2023 Danaf, da Silveira Scarpellini and Montandon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulator of G-Protein Signaling (RGS) Protein Modulation of Opioid Receptor Signaling as a Potential Target for Pain Management.Front Mol Neurosci. 2020 Jan 24;13:5. doi: 10.3389/fnmol.2020.00005. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32038168 Free PMC article. Review.
-
Mu opioid receptor activation enhances regulator of G protein signaling 4 association with the mu opioid receptor/G protein complex in a GTP-dependent manner.J Neurochem. 2015 Oct;135(1):76-87. doi: 10.1111/jnc.13222. Epub 2015 Jul 16. J Neurochem. 2015. PMID: 26119705 Free PMC article.
-
Mice Expressing Regulators of G protein Signaling-insensitive Gαo Define Roles of μ Opioid Receptor Gαo and Gαi Subunit Coupling in Inhibition of Presynaptic GABA Release.Mol Pharmacol. 2021 Sep;100(3):217-223. doi: 10.1124/molpharm.121.000249. Epub 2021 Jun 16. Mol Pharmacol. 2021. PMID: 34135098 Free PMC article.
-
μ-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology.Drug Alcohol Depend. 2012 Mar 1;121(3):173-80. doi: 10.1016/j.drugalcdep.2011.10.027. Epub 2011 Nov 29. Drug Alcohol Depend. 2012. PMID: 22129844 Free PMC article. Review.
-
Fentanyl-Induced Respiratory Depression and Locomotor Hyperactivity Are Mediated by μ-Opioid Receptors Expressed in Somatostatin-Negative Neurons.eNeuro. 2023 Jun 28;10(6):ENEURO.0035-23.2023. doi: 10.1523/ENEURO.0035-23.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37364996 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

