Treatment for ovarian clear cell carcinoma with combined inhibition of WEE1 and ATR
- PMID: 37087441
- PMCID: PMC10122390
- DOI: 10.1186/s13048-023-01160-y
Treatment for ovarian clear cell carcinoma with combined inhibition of WEE1 and ATR
Abstract
Background: Standard platinum-based therapy for ovarian cancer is inefficient against ovarian clear cell carcinoma (OCCC). OCCC is a distinct subtype of epithelial ovarian cancer. OCCC constitutes 25% of ovarian cancers in East Asia (Japan, Korea, China, Singapore) and 6-10% in Europe and North America. The cancer is characterized by frequent inactivation of ARID1A and 10% of cases of endometriosis progression to OCCC. The aim of this study was to identify drugs that are either FDA-approved or in clinical trials for the treatment of OCCC.
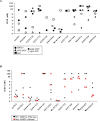
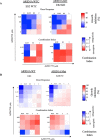
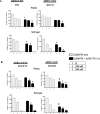
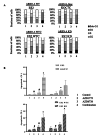
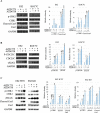
Results: High throughput screening of 166 compounds that are either FDA-approved, in clinical trials or are in pre-clinical studies identified several cytotoxic compounds against OCCC. ARID1A knockdown cells were more sensitive to inhibitors of either mTOR (PP242), dual mTOR/PI3K (GDC0941), ATR (AZD6738) or MDM2 (RG7388) compared to control cells. Also, compounds targeting BH3 domain (AZD4320) and SRC (AZD0530) displayed preferential cytotoxicity against ARID1A mutant cell lines. In addition, WEE1 inhibitor (AZD1775) showed broad cytotoxicity toward OCCC cell lines, irrespective of ARID1A status.
Conclusions: In a selection of 166 compounds we showed that inhibitors of ATR and WEE1 were cytotoxic against a panel of OCCC cell lines. These two drugs are already in other clinical trials, making them ideal candidates for treatment of OCCC.
Keywords: ATR; Ovarian clear cell carcinoma; WEE1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Therapeutic preferability of gemcitabine for ARID1A-deficient ovarian clear cell carcinoma.Gynecol Oncol. 2019 Dec;155(3):489-498. doi: 10.1016/j.ygyno.2019.10.002. Epub 2019 Oct 8. Gynecol Oncol. 2019. PMID: 31604667
-
The PI3K/mTOR dual inhibitor NVP-BEZ235 reduces the growth of ovarian clear cell carcinoma.Oncol Rep. 2014 Aug;32(2):553-8. doi: 10.3892/or.2014.3268. Epub 2014 Jun 13. Oncol Rep. 2014. PMID: 24927217
-
Low-dose triple drug combination targeting the PI3K/AKT/mTOR pathway and the MAPK pathway is an effective approach in ovarian clear cell carcinoma.Cancer Lett. 2019 Oct 1;461:102-111. doi: 10.1016/j.canlet.2019.07.004. Epub 2019 Jul 15. Cancer Lett. 2019. PMID: 31319139
-
Precision medicine for ovarian clear cell carcinoma based on gene alterations.Int J Clin Oncol. 2020 Mar;25(3):419-424. doi: 10.1007/s10147-020-01622-z. Epub 2020 Feb 4. Int J Clin Oncol. 2020. PMID: 32020380 Review.
-
Clinical analysis and literature review of a case of ovarian clear cell carcinoma with PIK3CA gene mutation: A case report.Medicine (Baltimore). 2022 Sep 16;101(37):e30666. doi: 10.1097/MD.0000000000030666. Medicine (Baltimore). 2022. PMID: 36123851 Free PMC article. Review.
Cited by
-
Aberrant SWI/SNF Complex Members Are Predominant in Rare Ovarian Malignancies-Therapeutic Vulnerabilities in Treatment-Resistant Subtypes.Cancers (Basel). 2024 Sep 3;16(17):3068. doi: 10.3390/cancers16173068. Cancers (Basel). 2024. PMID: 39272926 Free PMC article. Review.
-
Harnessing machine learning to find synergistic combinations for FDA-approved cancer drugs.Sci Rep. 2024 Jan 29;14(1):2428. doi: 10.1038/s41598-024-52814-w. Sci Rep. 2024. PMID: 38287066 Free PMC article.
-
Endometriosis-Associated Ovarian Carcinomas: How PI3K/AKT/mTOR Pathway Affects Their Pathogenesis.Biomolecules. 2023 Aug 16;13(8):1253. doi: 10.3390/biom13081253. Biomolecules. 2023. PMID: 37627318 Free PMC article. Review.
References
-
- Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88(11):2584–9. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

