Differential regulation of ATP hydrolysis of RIG-I-like receptors by transactivation response RNA-binding protein
- PMID: 37078499
- PMCID: PMC10170298
- DOI: 10.1042/BSR20222152
Differential regulation of ATP hydrolysis of RIG-I-like receptors by transactivation response RNA-binding protein
Abstract
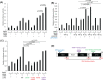
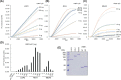
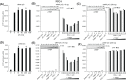
Retinoic acid inducible gene (RIG)-I-like receptors (RLRs), including RIG-I, melanoma differentiation associated-5 (MDA5), and laboratory of genetics and physiology 2 (LGP2), play pivotal roles in viral RNA sensing to initiate antiviral interferon (IFN) responses. We previously reported that an RNA-silencing regulator, transactivation response RNA-binding protein (TRBP), up-regulates MDA5/LGP2-mediated IFN responses through interaction with LGP2. Here, we aimed to investigate the mechanism underlying the TRBP-mediated up-regulation of IFN response. Data indicated that phosphomimetic TRBP showed a modest effect, whereas the nonphosphorylated form exhibited hyperactivity in enhancing Cardiovirus-triggered IFN responses. These results suggest that encephalomyocarditis virus (EMCV) attenuates the TRBP-mediated IFN response via TRBP phosphorylation, since EMCV infection activates the kinase responsible for TRBP phosphorylation for virus replication. Furthermore, we found that TRBP-mediated up-regulation of IFN response required the ATP hydrolysis and RNA binding of LGP2. TRBP enhanced RNA-dependent ATP hydrolysis by LGP2 but not that by RIG-I or MDA5. Nonphosphorylated TRBP exhibited higher levels of activity than phosphomimetic TRBP did, suggesting its possible involvement in the mechanism underlying the up-regulation of IFN response. TRBP activated the ATP hydrolysis of LGP2 and RIG-I, but not that of MDA5, in the absence of RNA. Collectively, we showed that TRBP differentially regulated RLR-mediated ATP hydrolysis. Further elucidation of the mechanism underlying the regulation of ATP hydrolysis leading to IFN response and self- and non-self-RNA discrimination could advance the development of effective therapeutic agents against autoimmune diseases.
Keywords: ATPase; RNA-binding proteins; host-pathogen interactions; innate immunity; interferons.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Effect of variants in LGP2 on MDA5-mediated activation of interferon response and suppression of hepatitis D virus replication.J Hepatol. 2023 Jan;78(1):78-89. doi: 10.1016/j.jhep.2022.08.041. Epub 2022 Sep 22. J Hepatol. 2023. PMID: 36152765
-
PACT is required for MDA5-mediated immunoresponses triggered by Cardiovirus infection via interaction with LGP2.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):227-233. doi: 10.1016/j.bbrc.2017.10.048. Epub 2017 Oct 12. Biochem Biophys Res Commun. 2017. PMID: 29032202
-
The TAR-RNA binding protein is required for immunoresponses triggered by Cardiovirus infection.Biochem Biophys Res Commun. 2016 Nov 11;480(2):187-193. doi: 10.1016/j.bbrc.2016.10.023. Epub 2016 Oct 13. Biochem Biophys Res Commun. 2016. PMID: 27743889 Free PMC article.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
-
RIG-I-like receptors: Molecular mechanism of activation and signaling.Adv Immunol. 2023;158:1-74. doi: 10.1016/bs.ai.2023.03.001. Epub 2023 May 9. Adv Immunol. 2023. PMID: 37453753 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

