Secretory-IgA binding to intestinal microbiota attenuates inflammatory reactions as the intestinal barrier of preterm infants matures
- PMID: 37070830
- PMCID: PMC10570995
- DOI: 10.1093/cei/uxad042
Secretory-IgA binding to intestinal microbiota attenuates inflammatory reactions as the intestinal barrier of preterm infants matures
Abstract
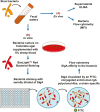
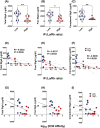
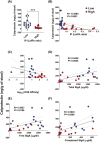
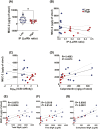
Previous work has shown that Secretory-IgA (SIgA) binding to the intestinal microbiota is variable and may regulate host inflammatory bowel responses. Nevertheless, the impact of the SIgA functional binding to the microbiota remains largely unknown in preterm infants whose immature epithelial barriers make them particularly susceptible to inflammation. Here, we investigated SIgA binding to intestinal microbiota isolated from stools of preterm infants <33 weeks gestation with various levels of intestinal permeability. We found that SIgA binding to intestinal microbiota attenuates inflammatory reactions in preterm infants. We also observed a significant correlation between SIgA affinity to the microbiota and the infant's intestinal barrier maturation. Still, SIgA affinity was not associated with developing host defenses, such as the production of mucus and inflammatory calprotectin protein, but it depended on the microbiota shifts as the intestinal barrier matures. In conclusion, we reported an association between the SIgA functional binding to the microbiota and the maturity of the preterm infant's intestinal barrier, indicating that the pattern of SIgA coating is altered as the intestinal barrier matures.
Trial registration: ClinicalTrials.gov NCT01756040.
Keywords: Secretory immunoglobulin A; human; intestinal permeability; intestine; microbiota.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Secretory immunoglobulin A in preterm infants: determination of normal values in breast milk and stool.Pediatr Res. 2022 Oct;92(4):979-986. doi: 10.1038/s41390-021-01930-8. Epub 2021 Dec 24. Pediatr Res. 2022. PMID: 34952939
-
Free and complexed-secretory immunoglobulin A triggers distinct intestinal epithelial cell responses.Clin Exp Immunol. 2016 Sep;185(3):338-47. doi: 10.1111/cei.12801. Epub 2016 Jul 26. Clin Exp Immunol. 2016. PMID: 27084834 Free PMC article.
-
Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells.Int J Mol Sci. 2020 Dec 4;21(23):9254. doi: 10.3390/ijms21239254. Int J Mol Sci. 2020. PMID: 33291586 Free PMC article. Review.
-
Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism.Immunol Lett. 2014 Dec;162(2 Pt A):10-21. doi: 10.1016/j.imlet.2014.05.008. Epub 2014 May 27. Immunol Lett. 2014. PMID: 24877874 Free PMC article. Review.
-
Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression.Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):3074-9. doi: 10.1073/pnas.1315792111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24569806 Free PMC article.
Cited by
-
Editorial: The synthesis of secretory immunoglobulin A in mucosal tissue: mucosal-associated invariant T, T follicular helper, and B cells.Front Immunol. 2024 Oct 17;15:1504432. doi: 10.3389/fimmu.2024.1504432. eCollection 2024. Front Immunol. 2024. PMID: 39483477 Free PMC article. No abstract available.
-
Dietary fiber pectin: challenges and potential anti-inflammatory benefits for preterms and newborns.Front Nutr. 2024 Jan 12;10:1286138. doi: 10.3389/fnut.2023.1286138. eCollection 2023. Front Nutr. 2024. PMID: 38283907 Free PMC article. Review.
-
Epigenetic regulation in epithelial cells and innate lymphocyte responses to S. Typhi infection: insights into IFN-γ production and intestinal immunity.Front Immunol. 2024 Sep 20;15:1448717. doi: 10.3389/fimmu.2024.1448717. eCollection 2024. Front Immunol. 2024. PMID: 39372404 Free PMC article.
-
Role of cellular effectors in the induction and maintenance of IgA responses leading to protective immunity against enteric bacterial pathogens.Front Immunol. 2024 Sep 11;15:1446072. doi: 10.3389/fimmu.2024.1446072. eCollection 2024. Front Immunol. 2024. PMID: 39324143 Free PMC article. Review.
-
Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations.Biology (Basel). 2024 Apr 6;13(4):243. doi: 10.3390/biology13040243. Biology (Basel). 2024. PMID: 38666855 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

