Longitudinal analyses using 18F-Fluorodeoxyglucose positron emission tomography with computed tomography as a measure of COVID-19 severity in the aged, young, and humanized ACE2 SARS-CoV-2 hamster models
- PMID: 37068595
- PMCID: PMC10105383
- DOI: 10.1016/j.antiviral.2023.105605
Longitudinal analyses using 18F-Fluorodeoxyglucose positron emission tomography with computed tomography as a measure of COVID-19 severity in the aged, young, and humanized ACE2 SARS-CoV-2 hamster models
Abstract
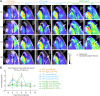
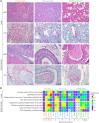
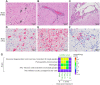
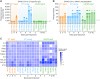
This study compared disease progression of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in three different models of golden hamsters: aged (≈60 weeks old) wild-type (WT), young (6 weeks old) WT, and adult (14-22 weeks old) hamsters expressing the human-angiotensin-converting enzyme 2 (hACE2) receptor. After intranasal (IN) exposure to the SARS-CoV-2 Washington isolate (WA01/2020), 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography with computed tomography (18F-FDG PET/CT) was used to monitor disease progression in near real time and animals were euthanized at pre-determined time points to directly compare imaging findings with other disease parameters associated with coronavirus disease 2019 (COVID-19). Consistent with histopathology, 18F-FDG-PET/CT demonstrated that aged WT hamsters exposed to 105 plaque forming units (PFU) developed more severe and protracted pneumonia than young WT hamsters exposed to the same (or lower) dose or hACE2 hamsters exposed to a uniformly lethal dose of virus. Specifically, aged WT hamsters presented with a severe interstitial pneumonia through 8 d post-exposure (PE), while pulmonary regeneration was observed in young WT hamsters at that time. hACE2 hamsters exposed to 100 or 10 PFU virus presented with a minimal to mild hemorrhagic pneumonia but succumbed to SARS-CoV-2-related meningoencephalitis by 6 d PE, suggesting that this model might allow assessment of SARS-CoV-2 infection on the central nervous system (CNS). Our group is the first to use (18F-FDG) PET/CT to differentiate respiratory disease severity ranging from mild to severe in three COVID-19 hamster models. The non-invasive, serial measure of disease progression provided by PET/CT makes it a valuable tool for animal model characterization.
Keywords: 2-Deoxy-2-[fluorine-18]fluoro-D-glucose ((18)F-FDG) PET/CT; Animal models; COVID-19; Computed tomography (CT); Hamsters; SARS-CoV-2.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Differences in Susceptibility to SARS-CoV-2 Infection Among Transgenic hACE2-Hamster Founder Lines.Viruses. 2024 Oct 17;16(10):1625. doi: 10.3390/v16101625. Viruses. 2024. PMID: 39459957 Free PMC article.
-
Hamsters Expressing Human Angiotensin-Converting Enzyme 2 Develop Severe Disease following Exposure to SARS-CoV-2.mBio. 2022 Feb 22;13(1):e0290621. doi: 10.1128/mbio.02906-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073750 Free PMC article.
-
124I-Iodo-DPA-713 Positron Emission Tomography in a Hamster Model of SARS-CoV-2 Infection.Mol Imaging Biol. 2022 Feb;24(1):135-143. doi: 10.1007/s11307-021-01638-5. Epub 2021 Aug 23. Mol Imaging Biol. 2022. PMID: 34424479 Free PMC article.
-
Hamsters as a Model of Severe Acute Respiratory Syndrome Coronavirus-2.Comp Med. 2021 Oct 1;71(5):398-410. doi: 10.30802/AALAS-CM-21-000036. Epub 2021 Sep 29. Comp Med. 2021. PMID: 34588095 Free PMC article. Review.
-
18F-FDG-PET/CT in SARS-CoV-2 infection and its sequelae.Rev Esp Med Nucl Imagen Mol (Engl Ed). 2021 Sep-Oct;40(5):299-309. doi: 10.1016/j.remnie.2021.07.005. Epub 2021 Jul 28. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2021. PMID: 34340958 Free PMC article. Review.
Cited by
-
Differences in Susceptibility to SARS-CoV-2 Infection Among Transgenic hACE2-Hamster Founder Lines.Viruses. 2024 Oct 17;16(10):1625. doi: 10.3390/v16101625. Viruses. 2024. PMID: 39459957 Free PMC article.
-
Characterization of therapeutic antibody efficacy against multiple SARS-CoV-2 variants in the hamster model.Antiviral Res. 2024 Oct;230:105987. doi: 10.1016/j.antiviral.2024.105987. Epub 2024 Aug 13. Antiviral Res. 2024. PMID: 39147143
References
-
- Abers M.S., Delmonte O.M., Ricotta E.E., Fintzi J., Fink D.L., de Jesus A.A.A., Zarember K.A., Alehashemi S., Oikonomou V., Desai J.V., Canna S.W., Shakoory B., Dobbs K., Imberti L., Sottini A., Quiros-Roldan E., Castelli F., Rossi C., Brugnoni D., Biondi A., Bettini L.R., D'Angio’ M., Bonfanti P., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Gliniewicz E.F., Shaw E., Kahle D.E., Rastegar A.T., Stack M., Myint-Hpu K., Levinson S.L., DiNubile M.J., Chertow D.W., Burbelo P.D., Cohen J.I., Calvo K.R., Tsang J.S., Su H.C., Gallin J.I., Kuhns D.B., Goldbach-Mansky R., Lionakis M.S., Notarangelo L.D. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144455. - DOI - PMC - PubMed
-
- Bai Y., Xu J., Chen L., Fu C., Kang Y., Zhang W., Fakhri G.E., Gu J., Shao F., Wang M. Inflammatory response in lungs and extrapulmonary sites detected by [(18)F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur. J. Nucl. Med. Mol. Imag. 2021;48:2531–2542. doi: 10.1007/s00259-020-05083-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

