Linking dysbiosis to precancerous stomach through inflammation: Deeper than and beyond imaging
- PMID: 37063848
- PMCID: PMC10102473
- DOI: 10.3389/fimmu.2023.1134785
Linking dysbiosis to precancerous stomach through inflammation: Deeper than and beyond imaging
Abstract
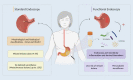
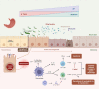
Upper gastrointestinal endoscopy is considered the gold standard for gastric lesions detection and surveillance, but it is still associated with a non-negligible rate of missing conditions. In the Era of Personalized Medicine, biomarkers could be the key to overcome missed lesions or to better predict recurrence, pushing the frontier of endoscopy to functional endoscopy. In the last decade, microbiota in gastric cancer has been extensively explored, with gastric carcinogenesis being associated with progressive dysbiosis. Helicobacter pylori infection has been considered the main causative agent of gastritis due to its interference in disrupting the acidic environment of the stomach through inflammatory mediators. Thus, does inflammation bridge the gap between gastric dysbiosis and the gastric carcinogenesis cascade and could the microbiota-inflammation axis-derived biomarkers be the answer to the unmet challenge of functional upper endoscopy? To address this question, in this review, the available evidence on the role of gastric dysbiosis and chronic inflammation in precancerous conditions of the stomach is summarized, particularly targeting the nuclear factor-κB (NF-κB), toll-like receptors (TLRs) and cyclooxygenase-2 (COX-2) pathways. Additionally, the potential of liquid biopsies as a non-invasive source and the clinical utility of studied biomarkers is also explored. Overall, and although most studies offer a mechanistic perspective linking a strong proinflammatory Th1 cell response associated with, but not limited to, chronic infection with Helicobacter pylori, promising data recently published highlights not only the diagnostic value of microbial biomarkers but also the potential of gastric juice as a liquid biopsy pushing forward the concept of functional endoscopy and personalized care in gastric cancer early diagnosis and surveillance.
Keywords: atrophic gastritis; biomarkers; functional endoscopy; inflammation; microbiota.
Copyright © 2023 Lopes, Almeida, Pimentel-Nunes, Dinis-Ribeiro and Pereira.
Conflict of interest statement
MD-R receives funding from Fujifilm through a scopes loan. The other authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Probiotics mitigate Helicobacter pylori-induced gastric inflammation and premalignant lesions in INS-GAS mice with the modulation of gastrointestinal microbiota.Helicobacter. 2022 Aug;27(4):e12898. doi: 10.1111/hel.12898. Epub 2022 May 9. Helicobacter. 2022. PMID: 35531615
-
Gastric Microbiota in a Low-Helicobacter pylori Prevalence General Population and Their Associations With Gastric Lesions.Clin Transl Gastroenterol. 2020 Jul;11(7):e00191. doi: 10.14309/ctg.0000000000000191. Clin Transl Gastroenterol. 2020. PMID: 32764211 Free PMC article.
-
Suicide journey of H. pylori through gastric carcinogenesis: the role of non-H. pylori microbiome and potential consequences for clinical practice.Eur J Clin Microbiol Infect Dis. 2019 Sep;38(9):1591-1597. doi: 10.1007/s10096-019-03564-5. Epub 2019 Apr 17. Eur J Clin Microbiol Infect Dis. 2019. PMID: 31114971 Review.
-
Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study.Medicine (Baltimore). 2018 Jan;97(4):e9734. doi: 10.1097/MD.0000000000009734. Medicine (Baltimore). 2018. PMID: 29369216 Free PMC article.
-
Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis.World J Gastroenterol. 2015 Dec 7;21(45):12742-56. doi: 10.3748/wjg.v21.i45.12742. World J Gastroenterol. 2015. PMID: 26668499 Free PMC article. Review.
Cited by
-
Microbes translocation from oral cavity to nasopharyngeal carcinoma in patients.Nat Commun. 2024 Feb 22;15(1):1645. doi: 10.1038/s41467-024-45518-2. Nat Commun. 2024. PMID: 38388556 Free PMC article.
-
Exploring the association between inflammatory biomarkers and gastric cancer development: A two-sample mendelian randomization analysis.Medicine (Baltimore). 2024 Feb 2;103(5):e36458. doi: 10.1097/MD.0000000000036458. Medicine (Baltimore). 2024. PMID: 38306562 Free PMC article.
-
The Role of Probiotics in the Eradication of Helicobacter pylori and Overall Impact on Management of Peptic Ulcer: A Study Involving Patients Undergoing Triple Therapy in Bangladesh.Cureus. 2024 Mar 16;16(3):e56283. doi: 10.7759/cureus.56283. eCollection 2024 Mar. Cureus. 2024. PMID: 38495972 Free PMC article.
-
Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort.Cancers (Basel). 2023 Aug 4;15(15):3974. doi: 10.3390/cancers15153974. Cancers (Basel). 2023. PMID: 37568790 Free PMC article.
-
Histopathological staging of atrophic lesions of gastric mucosa.Heliyon. 2024 Mar 14;10(6):e27845. doi: 10.1016/j.heliyon.2024.e27845. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38560685 Free PMC article.
References
-
- Ferlay J LM, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. . Global cancer observatory: Cancer tomorrow (2020). Available at: https://gco.iarc.fr/tomorrow.
-
- Correa P. Human gastric carcinogenesis: A multistep and multifactorial process- first american cancer society award lecture on cancer epidemiology and prevention. Cancer Res (1992) 52(24):6735–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

