Resolving Histological Inflammation in Ulcerative Colitis With Mirikizumab in the LUCENT Induction and Maintenance Trial Programmes
- PMID: 37057827
- PMCID: PMC10588772
- DOI: 10.1093/ecco-jcc/jjad050
Resolving Histological Inflammation in Ulcerative Colitis With Mirikizumab in the LUCENT Induction and Maintenance Trial Programmes
Abstract
Background and aims: To evaluate the effect of mirikizumab, a p19-targeted anti-interleukin-23, on histological and/or endoscopic outcomes in moderately-to-severely active ulcerative colitis [UC].
Methods: Endoscopic remission [ER], histological improvement [HI], histological remission [HR], histological-endoscopic mucosal improvement [HEMI], and histological-endoscopic mucosal remission [HEMR] were assessed at Week [W]12 [LUCENT-1: N = 1162, induction] and W40 [LUCENT-2: N = 544, maintenance] for patients randomised to mirikizumab or placebo. Analyses were performed to evaluate predictors of: HEMI at W12 with mirikizumab and HEMR at W40 in patients re-randomised to subcutaneous [SC] mirikizumab; associations between W12 histological/endoscopic endpoints and W40 outcomes in mirikizumab responders re-randomised to mirikizumab SC; and associations between W40 endoscopic normalisation [EN] with/without HR.
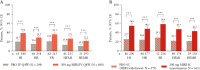
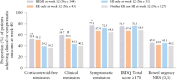
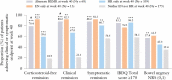
Results: Significantly more patients treated with mirikizumab achieved HI, HR, ER, HEMI, and HEMR vs placebo [p <0.001], irrespective of prior biologic/tofacitinib failure [p <0.05]. Lower clinical baseline disease activity, female sex, no baseline immunomodulator use, and no prior biologic/tofacitinib failure were predictors of HEMI at W12 [p <0.05]. Corticosteroid use and longer disease duration were negative predictors of achieving HEMR at W40 [p <0.05]. W12 HI, HR, or ER was associated with W40 HEMI or HEMR [p <0.05]; ER at W12 was associated with clinical remission [CR] [p <0.05] and corticosteroid-free remission [CSFR] at W40 [p = 0.052]. HR and HEMR at W12 were associated with CSFR, CR, and symptomatic remission at W40. Alternate HEMR [EN + HR] at W40 was associated with bowel urgency remission at W40 [p <0.05].
Conclusions: Early resolution of endoscopic and histological inflammation with mirikizumab is associated with better UC outcomes. Clinicaltrials.gov: LUCENT-1, NCT03518086; LUCENT-2, NCT03524092.
Keywords: Histological remission; mirikizumab; ulcerative colitis.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
FM has served as a speaker and received honoraria from: AbbVie, Biogen, Falk, Ferring, Hospira, Laboratórios Vitória, Merck Sharp & Dohme, and Vifor. RKP has receiving consulting fees from: AbbVie, Alimentiv, Allergan, Eli Lilly, Genentech, and PathAI. TK has received grants and/or contracts from: AbbVie, Activaid, Alfresa Pharma, Bristol-Myers Squibb, EA Pharma, Eli Lilly Japan, Gilead Sciences, Google Asia Pacific, Janssen Japan, JIMRO, JMDC, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Pfizer Japan, Takeda, and Zeria Pharmaceutical; has received lecture payments or honoraria from: AbbVie, Activaid, Alfresa Pharma, Galapagos, Janssen Japan, JIMRO, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Kayaku, Pfizer Japan, Takeda, ThermoFisher Diagnostics, Zeria Pharmaceutical; has received payment for expert testimony from: AbbVie, Activaid, Alfresa Pharma, EA Pharma, Janssen Japan, KISSEI Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe, Mochida Pharmaceutical, Nippon Kayaku, Pfizer Japan, and Takeda; and has participated on data safety monitoring boards or advisory boards for: Bristol-Myers Squibb, EA Pharma, Eli Lilly, and Janssen. VJ has received grants or contracts from: AbbVie, Adare, Atlantic, Boehringer Ingelheim, Celgene/Bristol-Myers Squibb, Eli Lilly, Janssen, Seres, Takeda, UCB Pharma, and VH Squared; has received consulting fees from: AbbVie, Alimentiv, Arena, Asahi, Asieris, Bristol-Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Kabi, Kasei Pharma, Landos Biopharma, Merck, Mylan, Organon Pandion, Pendopharm, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Reistone Biopharma, Roche, Sandoz, Second Genome, Takeda, Teva, Ventyx Biosciences, and Vividion Therapeutics; has received payments or honoraria from: AbbVie, Ferring, Galapagos, Janssen, Pfizer, and Takeda; and has participated on data safety monitoring or advisory boards for: AbbVie, Arena, Bristol-Myers Squibb, Fresenius, Gilead Sciences, Janssen, Kabi, Mylan, Roche, and Takeda. FR has received consulting or advisory board fees from: AbbVie, Adnovate, Agomab, Allergan, Arena, Boehringer-Ingelheim, Celgene/BMS, CDISC, Celsius, Cowen, Ferring, Galapagos, Galmed, Genentech, Gilead Sciences, Gossamer, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Mopac, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB Pharma, Ysios, and 89Bio. IR, TL, NM, and MS are employees and shareholders of Eli Lilly and Company. MP has nothing to disclose. LPB has received consulting fees from: AbbVie, Abivax, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, Bristol-Myers Squibb, Celltrion, Connect Biopharma, Cytoki Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Gossamer Bio, H.A.C. Pharma, IAG, InDex Pharmaceuticals, Inotrem, Janssen, Medac, Mopac, Morphic, Merck Sharp & Dohme, Norgine, Novartis, OM Pharma, Ono Pharmaceutical, OSE Immunotherapeutics, Pandion Therapeutics, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Roche, Sandoz, Takeda, Theravance, ThermoFisher Diagnostics, TiGenix, Tillotts Pharma, Viatris, Vifor, and YSOPIA Bioscience; has received grants from: Celltrion, Fresenius Kabi, and Takeda; and has received lecture fees from: AbbVie, Amgen, Arena, Biogen, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Genentech, Gilead Sciences, Janssen, Medac, Merck Sharp & Dohme, Pfizer, Sandoz, Takeda, Tillotts Pharma, Viatris, and Vifor.
Figures
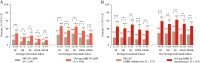

Similar articles
-
Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study.Inflamm Bowel Dis. 2024 Oct 25:izae253. doi: 10.1093/ibd/izae253. Online ahead of print. Inflamm Bowel Dis. 2024. PMID: 39448057
-
Mirikizumab Improves Quality of Life in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From the Phase 3 LUCENT-1 Induction and LUCENT-2 Maintenance Studies.Crohns Colitis 360. 2023 Nov 7;5(4):otad070. doi: 10.1093/crocol/otad070. eCollection 2023 Oct. Crohns Colitis 360. 2023. PMID: 38034882 Free PMC article.
-
Association of Bowel Urgency With Quality-of-Life Measures in Patients With Moderately-to-Severely Active Ulcerative Colitis: Results From Phase 3 LUCENT-1 (Induction) and LUCENT-2 (Maintenance) Studies.Crohns Colitis 360. 2024 Jan 6;6(1):otae001. doi: 10.1093/crocol/otae001. eCollection 2024 Jan. Crohns Colitis 360. 2024. PMID: 38313767 Free PMC article.
-
Mirikizumab: A New Therapeutic Option for the Treatment of Ulcerative Colitis.Ann Pharmacother. 2024 Nov;58(11):1134-1139. doi: 10.1177/10600280241229742. Epub 2024 Feb 12. Ann Pharmacother. 2024. PMID: 38344998 Review.
-
Efficacy and safety of mirikizumab compared with currently approved biologic drugs for the treatment of ulcerative colitis: A systematic review and network meta-analysis.Pharmacotherapy. 2024 Oct;44(10):811-821. doi: 10.1002/phar.4611. Epub 2024 Sep 25. Pharmacotherapy. 2024. PMID: 39320112 Review.
Cited by
-
AI and endoscopy/histology in UC: the rise of machine.Therap Adv Gastroenterol. 2024 Oct 18;17:17562848241275294. doi: 10.1177/17562848241275294. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39435049 Free PMC article. Review.
-
Anti-IL23/12 agents and JAK inhibitors for inflammatory bowel disease.Front Immunol. 2024 Jul 17;15:1393463. doi: 10.3389/fimmu.2024.1393463. eCollection 2024. Front Immunol. 2024. PMID: 39086483 Free PMC article. Review.
-
Mirikizumab: First Approval.Drugs. 2023 Jul;83(11):1045-1052. doi: 10.1007/s40265-023-01909-1. Drugs. 2023. PMID: 37389706 Review.
References
-
- Kobayashi T, Siegmund B, Le Berre C, et al. . Ulcerative colitis. Nat Rev Dis Primers 2020;6:74. - PubMed
-
- Ho EY, Cominelli F, Katz J.. Ulcerative colitis: what is the optimal treatment goal and how do we achieve it? Curr Treat Options Gastroenterol 2015;13:130–42. - PubMed
-
- D’Haens G, Sandborn WJ, Feagan BG, et al. . A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–86. - PubMed
-
- Travis SP, Higgins PDR, Orchard T, et al. . Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther 2011;34:113–24. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. .; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

