Porcine Epidemic Diarrhea Virus Antagonizes Host IFN-λ-Mediated Responses by Tilting Transcription Factor STAT1 toward Acetylation over Phosphorylation To Block Its Activation
- PMID: 37052505
- PMCID: PMC10294651
- DOI: 10.1128/mbio.03408-22
Porcine Epidemic Diarrhea Virus Antagonizes Host IFN-λ-Mediated Responses by Tilting Transcription Factor STAT1 toward Acetylation over Phosphorylation To Block Its Activation
Abstract
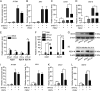
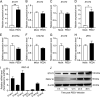
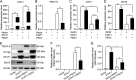
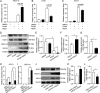
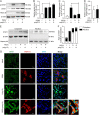
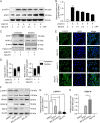
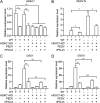
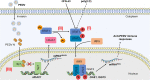
Porcine epidemic diarrhea virus (PEDV) is the main etiologic agent causing acute swine epidemic diarrhea, leading to severe economic losses to the pig industry. PEDV has evolved to deploy complicated antagonistic strategies to escape from host antiviral innate immunity. Our previous study demonstrated that PEDV downregulates histone deacetylase 1 (HDAC1) expression by binding viral nucleocapsid (N) protein to the transcription factor Sp1, inducing enhanced protein acetylation. We hypothesized that PEDV inhibition of HDAC1 expression would enhance acetylation of the molecules critical in innate immune signaling. Signal transducer and activator of transcription 1 (STAT1) is a crucial transcription factor regulating expression of interferon (IFN)-stimulated genes (ISGs) and anti-PEDV immune responses, as shown by overexpression, chemical inhibition, and gene knockdown in IPEC-J2 cells. We further show that PEDV infection and its N protein overexpression, although they upregulated STAT1 transcription level, could significantly block poly(I·C) and IFN-λ3-induced STAT1 phosphorylation and nuclear localization. Western blotting revealed that PEDV and its N protein promote STAT1 acetylation via downregulation of HDAC1. Enhanced STAT1 acetylation due to HDAC1 inhibition by PEDV or MS-275 (an HDAC1 inhibitor) impaired STAT1 phosphorylation, indicating that STAT1 acetylation negatively regulated its activation. These results, together with our recent report on PEDV N-mediated inhibition of Sp1, clearly indicate that PEDV manipulates the Sp1-HDAC1-STAT1 signaling axis to inhibit transcription of OAS1 and ISG15 in favor of its replication. This novel immune evasion mechanism is realized by suppression of STAT1 activation through preferential modulation of STAT1 acetylation over phosphorylation as a result of HDAC1 expression inhibition. IMPORTANCE PEDV has developed sophisticated evasion mechanisms to escape host IFN signaling via its structural and nonstructural proteins. STAT1 is one of the key transcription factors in regulating expression of ISGs. We found that PEDV and its N protein inhibit STAT1 phosphorylation and nuclear localization via inducing STAT1 acetylation as a result of HDAC1 downregulation, which, in turn, dampens the host IFN signaling activation. Our study demonstrates a novel mechanism that PEDV evades host antiviral innate immunity through manipulating the reciprocal relationship of STAT1 acetylation and phosphorylation. This provides new insights into the pathogenetic mechanisms of PEDV and even other coronaviruses.
Keywords: acetylation; histone deacetylase 1; interferon-stimulated genes; porcine epidemic diarrhea virus; signal transducer and activator of transcription 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Porcine Epidemic Diarrhea Virus Inhibits HDAC1 Expression To Facilitate Its Replication via Binding of Its Nucleocapsid Protein to Host Transcription Factor Sp1.J Virol. 2021 Aug 25;95(18):e0085321. doi: 10.1128/JVI.00853-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34232065 Free PMC article.
-
The death domain-associated protein suppresses porcine epidemic diarrhea virus replication by interacting with signal transducer and activator of transcription 1 and inducing downstream ISG15 expression.Vet Microbiol. 2024 May;292:110065. doi: 10.1016/j.vetmic.2024.110065. Epub 2024 Mar 30. Vet Microbiol. 2024. PMID: 38564904
-
Screening Host Antiviral Proteins under the Enhanced Immune Responses Induced by a Variant Strain of Porcine Epidemic Diarrhea Virus.Microbiol Spectr. 2022 Aug 31;10(4):e0066122. doi: 10.1128/spectrum.00661-22. Epub 2022 Jun 28. Microbiol Spectr. 2022. PMID: 35762780 Free PMC article.
-
Porcine epidemic diarrhea virus: A review of detection, inhibition of host gene expression and evasion of host innate immune.Microb Pathog. 2024 Oct;195:106873. doi: 10.1016/j.micpath.2024.106873. Epub 2024 Aug 21. Microb Pathog. 2024. PMID: 39173850 Review.
-
Porcine Epidemic Diarrhea Virus and the Host Innate Immune Response.Pathogens. 2020 May 11;9(5):367. doi: 10.3390/pathogens9050367. Pathogens. 2020. PMID: 32403318 Free PMC article. Review.
Cited by
-
Comparative Review of the State of the Art in Research on the Porcine Epidemic Diarrhea Virus and SARS-CoV-2, Scope of Knowledge between Coronaviruses.Viruses. 2024 Feb 2;16(2):238. doi: 10.3390/v16020238. Viruses. 2024. PMID: 38400014 Free PMC article. Review.
-
Developing Next-Generation Live Attenuated Vaccines for Porcine Epidemic Diarrhea Using Reverse Genetic Techniques.Vaccines (Basel). 2024 May 19;12(5):557. doi: 10.3390/vaccines12050557. Vaccines (Basel). 2024. PMID: 38793808 Free PMC article. Review.
-
Broad antagonism of coronaviruses nsp5 to evade the host antiviral responses by cleaving POLDIP3.PLoS Pathog. 2023 Oct 6;19(10):e1011702. doi: 10.1371/journal.ppat.1011702. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37801439 Free PMC article.
-
Mechanisms of mesothelial cell response to viral infections: HDAC1-3 inhibition blocks poly(I:C)-induced type I interferon response and modulates the mesenchymal/inflammatory phenotype.Front Cell Infect Microbiol. 2024 Feb 27;14:1308362. doi: 10.3389/fcimb.2024.1308362. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38476167 Free PMC article.
-
Integrating network pharmacology with pharmacological research to elucidate the mechanism of modified Gegen Qinlian Decoction in treating porcine epidemic diarrhea.Sci Rep. 2024 Aug 15;14(1):18929. doi: 10.1038/s41598-024-70059-5. Sci Rep. 2024. PMID: 39147857 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
