CD31 as a probable responding and gate-keeping protein of the blood-brain barrier and the risk of Alzheimer's disease
- PMID: 37051650
- PMCID: PMC10291450
- DOI: 10.1177/0271678X231170041
CD31 as a probable responding and gate-keeping protein of the blood-brain barrier and the risk of Alzheimer's disease
Abstract
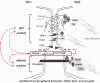
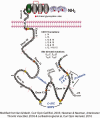
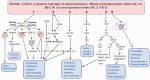
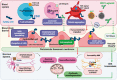
Several studies have shown that an abnormal vascular-immunity link could increase Alzheimer's disease (AD) risk; however, the mechanism is unclear. CD31, also named platelet endothelial cell adhesion molecule (PECAM), is a surface membrane protein of both endothelial and immune cells and plays important roles in the interaction between the vascular and immune systems. In this review, we focus on research regarding CD31 biological actions in the pathological process that may contribute to AD based on the following rationales. First, endothelial, leukocyte and soluble forms of CD31 play multi-roles in regulating transendothelial migration, increasing blood-brain barrier (BBB) permeability and resulting in neuroinflammation. Second, CD31 expressed by endothelial and immune cells dynamically modulates numbers of signaling pathways, including Src family kinases, selected G proteins, and β-catenin which in turn affect cell-matrix and cell-cell attachment, activation, permeability, survival, and ultimately neuronal cell injury. In endothelia and immune cells, these diverse CD31-mediated pathways act as a critical regulator in the immunity-endothelia-brain axis, thereby mediating AD pathogenesis in ApoE4 carriers, which is the major genetic risk factor for AD. This evidence suggests a novel mechanism and potential drug target for CD31 in the background of genetic vulnerabilities and peripheral inflammation for AD development and progression.
Keywords: Alzheimer's disease (AD); CD31; blood–brain barrier (BBB); neuroinflammation; trans-endothelia.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Monomeric C-reactive protein via endothelial CD31 for neurovascular inflammation in an ApoE genotype-dependent pattern: A risk factor for Alzheimer's disease?Aging Cell. 2021 Nov;20(11):e13501. doi: 10.1111/acel.13501. Epub 2021 Oct 23. Aging Cell. 2021. PMID: 34687487 Free PMC article.
-
PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation.Front Immunol. 2019 Apr 5;10:711. doi: 10.3389/fimmu.2019.00711. eCollection 2019. Front Immunol. 2019. PMID: 31024547 Free PMC article.
-
As human lung microvascular endothelia achieve confluence, src family kinases are activated, and tyrosine-phosphorylated p120 catenin physically couples NEU1 sialidase to CD31.Cell Signal. 2017 Jul;35:1-15. doi: 10.1016/j.cellsig.2017.03.014. Epub 2017 Mar 24. Cell Signal. 2017. PMID: 28343945
-
The blood-brain barrier in Alzheimer's disease.Neurobiol Dis. 2017 Nov;107:41-56. doi: 10.1016/j.nbd.2016.07.007. Epub 2016 Jul 15. Neurobiol Dis. 2017. PMID: 27425887 Free PMC article. Review.
-
ApoE4-mediated blood-brain barrier damage in Alzheimer's disease: Progress and prospects.Brain Res Bull. 2023 Jul;199:110670. doi: 10.1016/j.brainresbull.2023.110670. Epub 2023 May 22. Brain Res Bull. 2023. PMID: 37224887 Review.
Cited by
-
The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice.Biomedicines. 2024 Aug 1;12(8):1726. doi: 10.3390/biomedicines12081726. Biomedicines. 2024. PMID: 39200191 Free PMC article.
-
Minimally invasive soft tissue repair using shrunken scaffolds.Nat Commun. 2024 Aug 7;15(1):6739. doi: 10.1038/s41467-024-51248-2. Nat Commun. 2024. PMID: 39112538 Free PMC article.
-
Monocyte/macrophage-mediated venous thrombus resolution.Front Immunol. 2024 Jul 19;15:1429523. doi: 10.3389/fimmu.2024.1429523. eCollection 2024. Front Immunol. 2024. PMID: 39100675 Free PMC article. Review.
References
-
- Ballabh P, Braun A, Nedergaard M.The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

