Potential Therapeutic Value of the STING Inhibitors
- PMID: 37049889
- PMCID: PMC10096477
- DOI: 10.3390/molecules28073127
Potential Therapeutic Value of the STING Inhibitors
Abstract
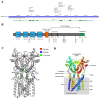
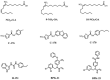
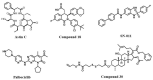
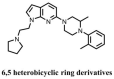
The stimulator of interferon genes (STING) is a critical protein in the activation of the immune system in response to DNA. It can participate the inflammatory response process by modulating the inflammation-preferred translation program through the STING-PKR-like endoplasmic reticulum kinase (PERK)-eIF2α pathway or by inducing the secretion of type I interferons (IFNs) and a variety of proinflammatory factors through the recruitment of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) or the regulation of the nuclear factor kappa-B (NF-κB) pathway. Based on the structure, location, function, genotype, and regulatory mechanism of STING, this review summarizes the potential value of STING inhibitors in the prevention and treatment of infectious diseases, psoriasis, systemic lupus erythematosus, non-alcoholic fatty liver disease, and other inflammatory and autoimmune diseases.
Keywords: STING; disease; genotype; inhibitors; innate immunity; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1.J Virol. 2014 May;88(10):5328-41. doi: 10.1128/JVI.00037-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24600004 Free PMC article.
-
TBK1 and IKKε Act Redundantly to Mediate STING-Induced NF-κB Responses in Myeloid Cells.Cell Rep. 2020 Apr 7;31(1):107492. doi: 10.1016/j.celrep.2020.03.056. Cell Rep. 2020. PMID: 32268090
-
TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections.Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2100225118. doi: 10.1073/pnas.2100225118. Proc Natl Acad Sci U S A. 2021. PMID: 33785602 Free PMC article.
-
Crosstalk and Prospects of TBK1 in Inflammation.Immunol Invest. 2024 Nov;53(8):1205-1233. doi: 10.1080/08820139.2024.2392587. Epub 2024 Aug 28. Immunol Invest. 2024. PMID: 39194013 Review.
-
[Innate immune DNA sensing pathways].Uirusu. 2014;64(1):83-94. doi: 10.2222/jsv.64.83. Uirusu. 2014. PMID: 25765984 Review. Japanese.
Cited by
-
Novel Therapies in APOL1-Mediated Kidney Disease: From Molecular Pathways to Therapeutic Options.Kidney Int Rep. 2023 Aug 29;8(11):2226-2234. doi: 10.1016/j.ekir.2023.08.028. eCollection 2023 Nov. Kidney Int Rep. 2023. PMID: 38025220 Free PMC article. Review.
-
The STING signaling pathways and bacterial infection.Apoptosis. 2024 Oct 20. doi: 10.1007/s10495-024-02031-7. Online ahead of print. Apoptosis. 2024. PMID: 39428409 Review.
-
Understanding nucleic acid sensing and its therapeutic applications.Exp Mol Med. 2023 Nov;55(11):2320-2331. doi: 10.1038/s12276-023-01118-6. Epub 2023 Nov 9. Exp Mol Med. 2023. PMID: 37945923 Free PMC article. Review.
-
VPS13C and STING expression in neuropsychiatric systemic lupus erythematosus: unveiling an unbreached territory.Lupus Sci Med. 2024 Sep 20;11(2):e001271. doi: 10.1136/lupus-2024-001271. Lupus Sci Med. 2024. PMID: 39306342 Free PMC article.
-
Regulation of cardiomyocyte intracellular trafficking and signal transduction by protein palmitoylation.Biochem Soc Trans. 2024 Feb 28;52(1):41-53. doi: 10.1042/BST20221296. Biochem Soc Trans. 2024. PMID: 38385554 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

