Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line
- PMID: 37049499
- PMCID: PMC10097357
- DOI: 10.3390/nu15071658
Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line
Abstract
Drug resistance is a well-known and significant obstacle in the battle against cancer, rendering chemotherapy treatments often ineffective. To improve the effectiveness of chemotherapy, researchers are exploring the use of natural molecules that can enhance its ability to kill cancer cells and limit their spread. Docosahexaenoic acid (DHA), a lipid found in marine fish, has been shown to enhance the cytotoxicity of various anti-cancer drugs in vitro and in vivo. While the combined use of chemotherapeutic drugs with DHA demonstrated promising preliminary results in clinical trials, there is still a significant amount of information to be discovered regarding the precise mechanism of action of DHA. As the biological pathways involved in the chemosensitization of already chemoresistant MCF-7 cells are still not entirely unraveled, in this study, we aimed to investigate whether DHA co-treatment could enhance the ability of the chemotherapy drug doxorubicin to inhibit the growth and invasion of MCF-7 breast cancer cells (MCF-7/Dox) that had become resistant to the drug. Upon treating MCF-7/Dox cells with DHA or DHA-doxorubicin, it was observed that the DHA-doxorubicin combination effectively enhanced cancer cell death by impeding in vitro propagation and invasive ability. In addition, it led to an increase in doxorubicin accumulation and triggered apoptosis by arresting the cell cycle at the G2/M phase. Other observed effects included a decrease in the multi-drug resistance (MDR) carrier P-glycoprotein (P-gp) and TG2, a tumor survival factor. Augmented quantities of molecules promoting apoptosis such as Bak1 and caspase-3 and enhanced lipid peroxidation were also detected. Our findings in the cell model suggest that DHA can be further investigated as a natural compound to be used alongside doxorubicin in the treatment of breast cancer that is unresponsive to chemotherapy.
Keywords: apoptosis; breast cancer; chemoresistance; chemosensitization; docosahexaenoic acid; drug accumulation; natural bioactive compound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
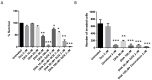
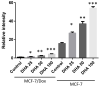
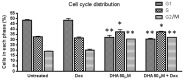
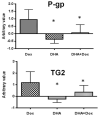

Similar articles
-
Docosahexaenoic Acid Incorporation Is Not Affected by Doxorubicin Chemotherapy in either Whole Cell or Lipid Raft Phospholipids of Breast Cancer Cells in vitro and Tumor Phospholipids in vivo.Lipids. 2020 Sep;55(5):549-565. doi: 10.1002/lipd.12252. Epub 2020 Jun 25. Lipids. 2020. PMID: 32588470
-
Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation.Free Radic Biol Med. 2005 Sep 15;39(6):742-51. doi: 10.1016/j.freeradbiomed.2005.04.023. Free Radic Biol Med. 2005. PMID: 16109304
-
DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA.Breast Cancer Res Treat. 2013 Oct;141(3):341-52. doi: 10.1007/s10549-013-2703-y. Epub 2013 Sep 24. Breast Cancer Res Treat. 2013. PMID: 24062211 Free PMC article.
-
The Potential of DHA as Cancer Therapy Strategies: A Narrative Review of In Vitro Cytotoxicity Trials.Nutrients. 2023 Apr 21;15(8):2006. doi: 10.3390/nu15082006. Nutrients. 2023. PMID: 37111226 Free PMC article. Review.
-
The role of peptides in reversing chemoresistance of breast cancer: current facts and future prospects.Front Pharmacol. 2023 May 22;14:1188477. doi: 10.3389/fphar.2023.1188477. eCollection 2023. Front Pharmacol. 2023. PMID: 37284316 Free PMC article. Review.
Cited by
-
Revolutionizing Cardiovascular Health with Nano Encapsulated Omega-3 Fatty Acids: A Nano-Solution Approach.Mar Drugs. 2024 May 30;22(6):256. doi: 10.3390/md22060256. Mar Drugs. 2024. PMID: 38921567 Free PMC article. Review.
-
Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies.Mar Drugs. 2024 Feb 28;22(3):114. doi: 10.3390/md22030114. Mar Drugs. 2024. PMID: 38535455 Free PMC article. Review.
References
-
- Sharmin S., Rahaman M.M., Martorell M., Sastre-Serra J., Sharifi-Rad J., Butnariu M., Bagiu I.C., Bagiu R.V., Islam M.T. Cytotoxicity of synthetic derivatives against breast cancer and multi-drug resistant breast cancer cell lines: A literature-based perspective study. Cancer Cell Int. 2021;21:612. doi: 10.1186/s12935-021-02309-9. - DOI - PMC - PubMed
-
- WHO . WHO: Breast Cancer. WHO; Geneva, Switzerland: 2021. World Health Organization (WHO) Report 2021.
-
- Liu H., Liu Y.Z., Zhang F., Wang H.S., Zhang G., Zhou B.H., Zuo Y.L., Cai S.H., Bu X.Z., Du J. Identification of potential pathways involved in the induction of cell cycle arrest and apoptosis by a new 4-arylidene curcumin analogue T63 in lung cancer cells: A comparative proteomic analysis. Mol. Biosyst. 2014;10:1320–1331. doi: 10.1039/c3mb70553f. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

