Role of GARP Vesicle Tethering Complex in Golgi Physiology
- PMID: 37047041
- PMCID: PMC10094427
- DOI: 10.3390/ijms24076069
Role of GARP Vesicle Tethering Complex in Golgi Physiology
Abstract
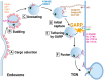
The Golgi associated retrograde protein complex (GARP) is an evolutionarily conserved component of Golgi membrane trafficking machinery that belongs to the Complexes Associated with Tethering Containing Helical Rods (CATCHR) family. Like other multisubunit tethering complexes such as COG, Dsl1, and Exocyst, the GARP is believed to function by tethering and promoting fusion of the endosome-derived small trafficking intermediate. However, even twenty years after its discovery, the exact structure and the functions of GARP are still an enigma. Recent studies revealed novel roles for GARP in Golgi physiology and identified human patients with mutations in GARP subunits. In this review, we summarized our knowledge of the structure of the GARP complex, its protein partners, GARP functions related to Golgi physiology, as well as cellular defects associated with the dysfunction of GARP subunits.
Keywords: GARP complex; Golgi; SNARE; glycosylation; vesicle tethering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cog5-Cog7 crystal structure reveals interactions essential for the function of a multisubunit tethering complex.Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15762-7. doi: 10.1073/pnas.1414829111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331899 Free PMC article.
-
The Caenorhabditis elegans GARP complex contains the conserved Vps51 subunit and is required to maintain lysosomal morphology.Mol Biol Cell. 2011 Jul 15;22(14):2564-78. doi: 10.1091/mbc.E10-06-0493. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613545 Free PMC article.
-
Golgi inCOGnito: From vesicle tethering to human disease.Biochim Biophys Acta Gen Subj. 2020 Nov;1864(11):129694. doi: 10.1016/j.bbagen.2020.129694. Epub 2020 Jul 27. Biochim Biophys Acta Gen Subj. 2020. PMID: 32730773 Free PMC article. Review.
-
Structure of a C-terminal fragment of its Vps53 subunit suggests similarity of Golgi-associated retrograde protein (GARP) complex to a family of tethering complexes.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14176-81. doi: 10.1073/pnas.1009419107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660722 Free PMC article.
-
Maintaining order: COG complex controls Golgi trafficking, processing, and sorting.FEBS Lett. 2019 Sep;593(17):2466-2487. doi: 10.1002/1873-3468.13570. Epub 2019 Aug 16. FEBS Lett. 2019. PMID: 31381138 Free PMC article. Review.
Cited by
-
Synergism of vesicle trafficking and cytoskeleton during regulation of plant growth and development: A mechanistic outlook.Heliyon. 2023 Nov 8;9(11):e21976. doi: 10.1016/j.heliyon.2023.e21976. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034654 Free PMC article. Review.
-
Classification and functional characterization of regulators of intracellular STING trafficking identified by genome-wide optical pooled screening.bioRxiv [Preprint]. 2024 Apr 9:2024.04.07.588166. doi: 10.1101/2024.04.07.588166. bioRxiv. 2024. Update in: Cell Syst. 2024 Dec 18;15(12):1264-1277.e8. doi: 10.1016/j.cels.2024.11.004 PMID: 38645119 Free PMC article. Updated. Preprint.
-
Golgi-associated retrograde protein (GARP) complex-dependent endosomes to trans Golgi network retrograde trafficking is controlled by Rab4b.Cell Mol Biol Lett. 2024 Apr 16;29(1):54. doi: 10.1186/s11658-024-00574-w. Cell Mol Biol Lett. 2024. PMID: 38627612 Free PMC article.
-
Acute GARP depletion disrupts vesicle transport, leading to severe defects in sorting, secretion, and O-glycosylation.bioRxiv [Preprint]. 2024 Oct 14:2024.10.07.617053. doi: 10.1101/2024.10.07.617053. bioRxiv. 2024. PMID: 39416116 Free PMC article. Preprint.
-
StaufenC facilitates utilization of the ERAD pathway to transport dsRNA through the endoplasmic reticulum to the cytosol.Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2322927121. doi: 10.1073/pnas.2322927121. Epub 2024 Jun 17. Proc Natl Acad Sci U S A. 2024. PMID: 38885386 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

