Contribution of Epstein-Barr Virus Lytic Proteins to Cancer Hallmarks and Implications from Other Oncoviruses
- PMID: 37046781
- PMCID: PMC10093119
- DOI: 10.3390/cancers15072120
Contribution of Epstein-Barr Virus Lytic Proteins to Cancer Hallmarks and Implications from Other Oncoviruses
Abstract
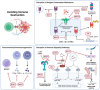
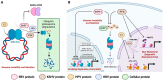
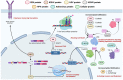
Epstein-Barr virus (EBV) is a prevalent human gamma-herpesvirus that infects the majority of the adult population worldwide and is associated with several lymphoid and epithelial malignancies. EBV displays a biphasic life cycle, namely, latent and lytic replication cycles, expressing a diversity of viral proteins. Among the EBV proteins being expressed during both latent and lytic cycles, the oncogenic roles of EBV lytic proteins are largely uncharacterized. In this review, the established contributions of EBV lytic proteins in tumorigenesis are summarized according to the cancer hallmarks displayed. We further postulate the oncogenic properties of several EBV lytic proteins by comparing the evolutionary conserved oncogenic mechanisms in other herpesviruses and oncoviruses.
Keywords: Epstein–Barr virus (EBV); cancer hallmarks; herpesvirus; lytic proteins; oncovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The lytic phase of Epstein-Barr virus plays an important role in tumorigenesis.Virus Genes. 2023 Feb;59(1):1-12. doi: 10.1007/s11262-022-01940-6. Epub 2022 Oct 15. Virus Genes. 2023. PMID: 36242711 Review.
-
Efficient Translation of Epstein-Barr Virus (EBV) DNA Polymerase Contributes to the Enhanced Lytic Replication Phenotype of M81 EBV.J Virol. 2018 Feb 26;92(6):e01794-17. doi: 10.1128/JVI.01794-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263273 Free PMC article.
-
Functional Implications of Epstein-Barr Virus Lytic Genes in Carcinogenesis.Cancers (Basel). 2022 Nov 24;14(23):5780. doi: 10.3390/cancers14235780. Cancers (Basel). 2022. PMID: 36497262 Free PMC article. Review.
-
Niclosamide inhibits lytic replication of Epstein-Barr virus by disrupting mTOR activation.Antiviral Res. 2017 Feb;138:68-78. doi: 10.1016/j.antiviral.2016.12.002. Epub 2016 Dec 8. Antiviral Res. 2017. PMID: 27939840
-
Epstein-Barr Virus BKRF4 Gene Product Is Required for Efficient Progeny Production.J Virol. 2017 Nov 14;91(23):e00975-17. doi: 10.1128/JVI.00975-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904200 Free PMC article.
References
-
- Chakravorty S., Yan B., Wang C., Wang L., Quaid J.T., Lin C.F., Briggs S.D., Majumder J., Canaria D.A., Chauss D., et al. Integrated Pan-Cancer Map of EBV-Associated Neoplasms Reveals Functional Host–Virus Interactions. Cancer Res. 2019;79:6010–6023. doi: 10.1158/0008-5472.CAN-19-0615. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

