Monoclonal antibodies for the treatment of squamous cell carcinoma: A literature review
- PMID: 37042307
- PMCID: PMC10172176
- DOI: 10.1002/cnr2.1802
Monoclonal antibodies for the treatment of squamous cell carcinoma: A literature review
Abstract
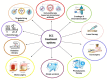
Background: Squamous cell carcinoma (SCC) is a relatively common and heterogenous malignancy of different organs, such as the skin, esophagus, and lungs. Although most cases experience good survival with surgical methods, management of advanced types of the disease remains challenging. Several modalities, including different chemotherapy regimens and immunotherapies, have been investigated in this matter, among which Monoclonal antibodies (Mabs) are one of the most promising ones. Since the development of Mabs, they have been widely used to treat different diseases. Mabs have shown significant efficacy with high specificity along with acceptable safety, which makes them a favorable option in cancer therapy. In this article, we aimed to review the different aspects of using Mabs in SCC therapy.
Recent findings: We found that treating with different Mabs has shown excellent efficacy accompanied by acceptable safety in treating SCC of different organs. Therefore, Mabs are considered great options in the treatment of SCC, especially in advanced cases. Overall, two highly potent types of Mabs in SCC therapy are anti-EGFR Mabs and checkpoint inhibitors, especially Cetuximab, Nimotuzumab, and PD-1 inhibitors. Bevacizumab is also a promising option as adjuvant therapy to other modalities.
Conclusion: Although some Mabs have shown promising outcomes in SCC therapy, their application as a part of cancer treatment depends on further investigations regarding cost-effectiveness and predictors of response. FDA has approved several Mabs in SCC therapies, and Mabs may have a crucial role in this era in the near future, especially in treating head and neck and esophageal SCC and metastatic lung cancer.
Keywords: anti-cancer; immunotherapy; monoclonal antibody; squamous cell carcinoma.
© 2023 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Treatment of unresectable squamous cell carcinoma of the skin with epidermal growth factor receptor antibodies--a case series.Eur J Dermatol. 2013 Sep-Oct;23(5):658-62. doi: 10.1684/ejd.2013.2153. Eur J Dermatol. 2013. PMID: 24135559
-
Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC).Cancer Treat Rev. 2014 May;40(4):567-77. doi: 10.1016/j.ctrv.2013.10.002. Epub 2013 Oct 12. Cancer Treat Rev. 2014. PMID: 24216225 Review.
-
Optical imaging predicts tumor response to anti-EGFR therapy.Cancer Biol Ther. 2010 Jul 15;10(2):166-71. doi: 10.4161/cbt.10.2.12164. Cancer Biol Ther. 2010. PMID: 20505368 Free PMC article.
-
Cetuximab in non-melanoma skin cancer.Expert Opin Biol Ther. 2012 Jul;12(7):949-56. doi: 10.1517/14712598.2012.681374. Epub 2012 Apr 23. Expert Opin Biol Ther. 2012. PMID: 22519406 Review.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
Cited by
-
Plant-Derived Polyphenols to Prevent and Treat Oral Mucositis Induced by Chemo- and Radiotherapy in Head and Neck Cancers Management.Cancers (Basel). 2024 Jan 6;16(2):260. doi: 10.3390/cancers16020260. Cancers (Basel). 2024. PMID: 38254751 Free PMC article. Review.
-
Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy.Biomedicines. 2024 Sep 23;12(9):2158. doi: 10.3390/biomedicines12092158. Biomedicines. 2024. PMID: 39335671 Free PMC article. Review.
-
Cetuximab chemotherapy resistance: Insight into the homeostatic evolution of head and neck cancer (Review).Oncol Rep. 2024 Jun;51(6):80. doi: 10.3892/or.2024.8739. Epub 2024 Apr 19. Oncol Rep. 2024. PMID: 38639184 Free PMC article. Review.
-
Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review.Cancers (Basel). 2024 Sep 14;16(18):3156. doi: 10.3390/cancers16183156. Cancers (Basel). 2024. PMID: 39335128 Free PMC article. Review.
References
-
- Squamous Cell Carcinoma ‐ Patient Information. 2018. http://skincancer.dermis.net/content/e04typesof/e151/e152/index_eng.html
-
- UK CR . Non‐melanoma skin cancer risk factors. 2018. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s...
-
- Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology e‐book. Elsevier Health Sciences; 2017.
-
- Biamonte F, Buffone C, Santamaria G, et al. Gene expression analysis of autofluorescence margins in leukoplakia and oral carcinoma: A pilot study. Oral Dis. 2021;27(2):193‐203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

