A Systematic Review of Second-Line Treatments in Antiviral Resistant Strains of HSV-1, HSV-2, and VZV
- PMID: 37041924
- PMCID: PMC10082683
- DOI: 10.7759/cureus.35958
A Systematic Review of Second-Line Treatments in Antiviral Resistant Strains of HSV-1, HSV-2, and VZV
Abstract
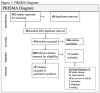
Drug-resistant variants of herpes simplex viruses (HSV) have been reported that are not effectively treated with first-line antiviral agents. The objective of this study was to evaluate available literature on the possible efficacy of second-line treatments in HSV and the use of second-line treatments in HSV strains that are resistant to first-line treatments. Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a final search was conducted in six databases on November 5, 2021 for all relevant literature using terms related to antiviral resistance, herpes, and HSV. Eligible manuscripts were required to report the presence of an existing or proposed second-line treatment for HSV-1, HSV-2, or varicella zoster virus (VZV); have full-text English-language access; and potentially reduce the rate of antiviral resistance. Following screening, 137 articles were included in qualitative synthesis. Of the included studies, articles that examined the relationship between viral resistance to first-line treatments and potential second-line treatments in HSV were included. The Cochrane risk-of-bias tool for randomized trials was used to assess risk of bias. Due to the heterogeneity of study designs, a meta-analysis of the studies was not performed. The dates in which accepted studies were published spanned from 2015-2021. In terms of sample characteristics, the majority (72.26%) of studies used Vero cells. When looking at the viruses on which the interventions were tested, the majority (84.67%) used HSV-1, with (34.31%) of these studies reporting testing on resistant HSV strains. Regarding the effectiveness of the proposed interventions, 91.97% were effective as potential managements for resistant strains of HSV. Of the papers reviewed, nectin in 2.19% of the reviews had efficacy as a second-line treatments in HSV, amenamevir in 2.19%, methanol extract in 2.19%, monoclonal antibodies in 1.46%, arbidol in 1.46%, siRNA swarms in 1.46%, Cucumis melo sulfated pectin in 1.46%, and components from Olea europeae in 1.46%. In addition to this griffithsin in 1.46% was effective, Morus alba L. in 1.46%, using nucleosides in 1.46%, botryosphaeran in 1.46%, monoterpenes in 1.46%, almond skin extracts in 1.46%, bortezomib in 1.46%, flavonoid compounds in 1.46%, andessential oils were effective in 1.46%, but not effective in 0.73%. The available literature reviewed consistently supports the existence and potentiality of second-line treatments for HSV strains that are resistant to first-line treatments. Immunocompromised patients have been noted to be the population most often affected by drug-resistant variants of HSV. Subsequently, we found that HSV infections in this patient population are challenging to manage clinically effectively. The goal of this systematic review is to provide additional information to patients on the potentiality of second-line treatment in HSV strains resistant to first-line treatments, especially those who are immunocompromised. All patients, whether they are immunocompromised or not, deserve to have their infections clinically managed in a manner supported by comprehensive research. This review provides necessary information about treatment options for patients with resistant HSV infections and their providers.
Keywords: anti-hsv; antiviral resistance; direct anti-viral agents; experimental pharmacology; general obgyn; hsv-1; hsv-2; resistant hsv; second line drugs; vzv.
Copyright © 2023, Lince et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Utility of ultra-deep sequencing for detection of varicella-zoster virus antiviral resistance mutations.Antiviral Res. 2018 Mar;151:20-23. doi: 10.1016/j.antiviral.2018.01.008. Epub 2018 Jan 12. Antiviral Res. 2018. PMID: 29337163
-
Antiviral efficacy of the helicase-primase inhibitor amenamevir in murine models of severe herpesvirus infection.Biochem Pharmacol. 2018 Dec;158:201-206. doi: 10.1016/j.bcp.2018.10.024. Epub 2018 Oct 24. Biochem Pharmacol. 2018. PMID: 30365949
-
Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management.Curr Opin Infect Dis. 2016 Dec;29(6):654-662. doi: 10.1097/QCO.0000000000000288. Curr Opin Infect Dis. 2016. PMID: 27306564 Review.
-
Public sector reforms and their impact on the level of corruption: A systematic review.Campbell Syst Rev. 2021 May 24;17(2):e1173. doi: 10.1002/cl2.1173. eCollection 2021 Jun. Campbell Syst Rev. 2021. PMID: 37131927 Free PMC article. Review.
Cited by
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
-
Oncolytic Virotherapy: A New Paradigm in Cancer Immunotherapy.Int J Mol Sci. 2024 Jan 18;25(2):1180. doi: 10.3390/ijms25021180. Int J Mol Sci. 2024. PMID: 38256250 Free PMC article. Review.
-
Bridging Flocculation of a Sterically Stabilized Cationic Latex as a Biosensor for the Detection of Microbial DNA after Amplification via PCR.Biomacromolecules. 2024 Mar 11;25(3):1629-1636. doi: 10.1021/acs.biomac.3c01187. Epub 2024 Feb 15. Biomacromolecules. 2024. PMID: 38361251 Free PMC article.
References
-
- Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: a single-center experience. El-Haddad D, El Chaer F, Vanichanan J, et al. Antiviral Res. 2016;134:58–62. - PubMed
-
- Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1. Lei Y, Chen W, Liang H, et al. Arch Virol. 2019;164:1259–1269. - PubMed
-
- Aminomethylnaphthoquinones and HSV-1: in vitro and in silico evaluations of potential antivirals. Pires de Mello CP, Sardoux NS, Terra L, et al. Antivir Ther. 2016;21:507–515. - PubMed
-
- Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. J Clin Epidemiol. 2009;62:1006–1012. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous