The SFTSV Nonstructural Proteins Induce Autophagy to Promote Viral Replication via Interaction with Vimentin
- PMID: 37039677
- PMCID: PMC10134822
- DOI: 10.1128/jvi.00302-23
The SFTSV Nonstructural Proteins Induce Autophagy to Promote Viral Replication via Interaction with Vimentin
Abstract
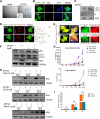
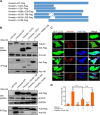
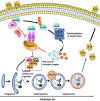
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly identified phlebovirus associated with severe hemorrhagic fever in humans. Studies have shown that SFTSV nucleoprotein (N) induces BECN1-dependent autophagy to promote viral assembly and release. However, the function of other SFTSV proteins in regulating autophagy has not been reported. In this study, we identify SFTSV NSs, a nonstructural protein that forms viroplasm-like structures in the cytoplasm of infected cells as the virus component mediating SFTSV-induced autophagy. We found that SFTSV NSs-induced autophagy was inclusion body independent, and most phenuivirus NSs had autophagy-inducing effects. Unlike N protein-induced autophagy, SFTSV NSs was key in regulating autophagy by interacting with the host's vimentin in an inclusion body-independent manner. NSs interacted with vimentin and induced vimentin degradation through the K48-linked ubiquitin-proteasome pathway. This negatively regulating Beclin1-vimentin complex formed and promoted autophagy. Furthermore, we identified the NSs-binding domain of vimentin and found that overexpression of wild-type vimentin antagonized the induced effect of NSs on autophagy and inhibited viral replication, suggesting that vimentin is a potential antiviral target. The present study shows a novel mechanism through which SFTSV nonstructural protein activates autophagy, which provides new insights into the role of NSs in SFTSV infection and pathogenesis. IMPORTANCE Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly emerging tick-borne pathogen that causes multifunctional organ failure and even death in humans. As a housekeeping mechanism for cells to maintain steady state, autophagy plays a dual role in viral infection and the host's immune response. However, the relationship between SFTSV infection and autophagy has not been described in detail yet. Here, we demonstrated that SFTSV infection induced complete autophagic flux and facilitated viral proliferation. We also identified a key mechanism underlying NSs-induced autophagy, in which NSs interacted with vimentin to inhibit the formation of the Beclin1-vimentin complex and induced vimentin degradation through K48-linked ubiquitination modification. These findings may help us understand the new functions and mechanisms of NSs and may aid in the identification of new antiviral targets.
Keywords: Beclin1-vimentin complex; IBs; NSs; SFTSV; autophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Severe Fever with Thrombocytopenia Syndrome Virus NSs Protein Interacts with CDK1 To Induce G2 Cell Cycle Arrest and Positively Regulate Viral Replication.J Virol. 2020 Feb 28;94(6):e01575-19. doi: 10.1128/JVI.01575-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852787 Free PMC article.
-
Nonstructural Protein NSs Activates Inflammasome and Pyroptosis through Interaction with NLRP3 in Human Microglial Cells Infected with Severe Fever with Thrombocytopenia Syndrome Bandavirus.J Virol. 2022 Jul 13;96(13):e0016722. doi: 10.1128/jvi.00167-22. Epub 2022 Jun 13. J Virol. 2022. PMID: 35695505 Free PMC article.
-
Two Conserved Amino Acids within the NSs of Severe Fever with Thrombocytopenia Syndrome Phlebovirus Are Essential for Anti-interferon Activity.J Virol. 2018 Sep 12;92(19):e00706-18. doi: 10.1128/JVI.00706-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021900 Free PMC article.
-
The Role of Non-Structural Protein NSs in the Pathogenesis of Severe Fever with Thrombocytopenia Syndrome.Viruses. 2021 May 11;13(5):876. doi: 10.3390/v13050876. Viruses. 2021. PMID: 34064604 Free PMC article. Review.
-
Unraveling the Underlying Interaction Mechanism Between Dabie bandavirus and Innate Immune Response.Front Immunol. 2021 May 27;12:676861. doi: 10.3389/fimmu.2021.676861. eCollection 2021. Front Immunol. 2021. PMID: 34122440 Free PMC article. Review.
Cited by
-
The Effect of Tryptophan-to-Tyrosine Mutation at Position 61 of the Nonstructural Protein of Severe Fever with Thrombocytopenia Syndrome Virus on Viral Replication through Autophagosome Modulation.Int J Mol Sci. 2024 Jun 10;25(12):6394. doi: 10.3390/ijms25126394. Int J Mol Sci. 2024. PMID: 38928101 Free PMC article.
-
Pathogenesis and virulence of Heartland virus.Virulence. 2024 Dec;15(1):2348252. doi: 10.1080/21505594.2024.2348252. Epub 2024 May 7. Virulence. 2024. PMID: 38712703 Free PMC article. Review.
-
Bunyavirus SFTSV NSs utilizes autophagy to escape the antiviral innate immune response.Autophagy. 2024 Oct;20(10):2133-2145. doi: 10.1080/15548627.2024.2356505. Epub 2024 Jun 30. Autophagy. 2024. PMID: 38762760
-
Current insights into human pathogenic phenuiviruses and the host immune system.Virulence. 2024 Dec;15(1):2384563. doi: 10.1080/21505594.2024.2384563. Epub 2024 Jul 29. Virulence. 2024. PMID: 39072499 Free PMC article. Review.
References
-
- Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 25:81–84. - PubMed
-
- Zhang YZ, Zhou DJ, Xiong Y, Chen XP, He YW, Sun Q, Yu B, Li J, Dai YA, Tian JH, Qin XC, Jin D, Cui Z, Luo XL, Li W, Lu S, Wang W, Peng JS, Guo WP, Li MH, Li ZJ, Zhang S, Chen C, Wang Y, de Jong MD, Xu J. 2011. Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi 32:209–220. - PubMed
-
- Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209:816–827. doi:10.1093/infdis/jit603. - DOI - PMC - PubMed
-
- Li H, Zhang LK, Li SF, Zhang SF, Wan WW, Zhang YL, Xin QL, Dai K, Hu YY, Wang ZB, Zhu XT, Fang YJ, Cui N, Zhang PH, Yuan C, Lu QB, Bai JY, Deng F, Xiao GF, Liu W, Peng K. 2019. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res 29:739–753. doi:10.1038/s41422-019-0214-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

