ISG15 Is Required for the Dissemination of Vaccinia Virus Extracellular Virions
- PMID: 37036376
- PMCID: PMC10269806
- DOI: 10.1128/spectrum.04508-22
ISG15 Is Required for the Dissemination of Vaccinia Virus Extracellular Virions
Abstract
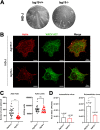
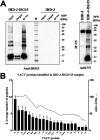
Viruses have developed many different strategies to counteract immune responses, and Vaccinia virus (VACV) is one of a kind in this aspect. To ensure an efficient infection, VACV undergoes a complex morphogenetic process resulting in the production of two types of infective virions: intracellular mature virus (MV) and extracellular enveloped virus (EV), whose spread depends on different dissemination mechanisms. MVs disseminate after cell lysis, whereas EVs are released or propelled in actin tails from living cells. Here, we show that ISG15 participates in the control of VACV dissemination. Infection of Isg15-/- mouse embryonic fibroblasts with VACV International Health Department-J (IHD-J) strain resulted in decreased EV production, concomitant with reduced induction of actin tails and the abolition of comet-shaped plaque formation, compared to Isg15+/+ cells. Transmission electron microscopy revealed the accumulation of intracellular virus particles and a decrease in extracellular virus particles in the absence of interferon-stimulated gene 15 (ISG15), a finding consistent with altered virus egress. Immunoblot and quantitative proteomic analysis of sucrose gradient-purified virions from both genotypes reported differences in protein levels and composition of viral proteins present on virions, suggesting an ISG15-mediated control of viral proteome. Lastly, the generation of a recombinant IHD-J expressing V5-tagged ISG15 (IHD-J-ISG15) allowed us to identify several viral proteins as potential ISG15 targets, highlighting the proteins A34 and A36, which are essential for EV formation. Altogether, our results indicate that ISG15 is an important host factor in the regulation of VACV dissemination. IMPORTANCE Viral infections are a constant battle between the virus and the host. While the host's only goal is victory, the main purpose of the virus is to spread and conquer new territories at the expense of the host's resources. Along millions of years of incessant encounters, poxviruses have developed a unique strategy consisting in the production two specialized "troops": intracellular mature virions (MVs) and extracellular virions (EVs). MVs mediate transmission between hosts, and EVs ensure advance on the battlefield mediating the long-range dissemination. The mechanism by which the virus "decides" to shed from the primary site of infection and its significant impact in viral transmission is not yet fully established. Here, we demonstrate that this process is finely regulated by ISG15/ISGylation, an interferon-induced ubiquitin-like protein with broad antiviral activity. Studying the mechanism that viruses use during infection could result in new ways of understanding our perpetual war against disease and how we might win the next great battle.
Keywords: ISG15; VACV; actin tails.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Engineered Promoter-Switched Viruses Reveal the Role of Poxvirus Maturation Protein A26 as a Negative Regulator of Viral Spread.J Virol. 2021 Sep 9;95(19):e0101221. doi: 10.1128/JVI.01012-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34260287 Free PMC article.
-
ISG15 Is a Novel Regulator of Lipid Metabolism during Vaccinia Virus Infection.Microbiol Spectr. 2022 Dec 21;10(6):e0389322. doi: 10.1128/spectrum.03893-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453897 Free PMC article.
-
Protein B5 is required on extracellular enveloped vaccinia virus for repulsion of superinfecting virions.J Gen Virol. 2012 Sep;93(Pt 9):1876-1886. doi: 10.1099/vir.0.043943-0. Epub 2012 May 23. J Gen Virol. 2012. PMID: 22622330 Free PMC article.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers.Vaccines (Basel). 2024 Feb 1;12(2):153. doi: 10.3390/vaccines12020153. Vaccines (Basel). 2024. PMID: 38400136 Free PMC article. Review.
References
-
- Nakashima H, Nguyen T, Goins WF, Chiocca EA. 2015. Interferon-stimulated gene 15 (ISG15) and ISG15-linked proteins can associate with members of the selective autophagic process, histone deacetylase 6 (HDAC6) and SQSTM1/p62. J Biol Chem 290:1485–1495. doi:10.1074/jbc.M114.593871. - DOI - PMC - PubMed
-
- Zhang X, Bogunovic D, Payelle-Brogard B, Francois-Newton V, Speer SD, Yuan C, Volpi S, Li Z, Sanal O, Mansouri D, Tezcan I, Rice GI, Chen C, Mansouri N, Mahdaviani SA, Itan Y, Boisson B, Okada S, Zeng L, Wang X, Jiang H, Liu W, Han T, Liu D, Ma T, Wang B, Liu M, Liu J-Y, Wang QK, Yalnizoglu D, Radoshevich L, Uzé G, Gros P, Rozenberg F, Zhang S-Y, Jouanguy E, Bustamante J, García-Sastre A, Abel L, Lebon P, Notarangelo LD, Crow YJ, Boisson-Dupuis S, Casanova J-L, Pellegrini S. 2015. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517:89–93. doi:10.1038/nature13801. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

