Heroin and its metabolites: relevance to heroin use disorder
- PMID: 37031205
- PMCID: PMC10082801
- DOI: 10.1038/s41398-023-02406-5
Heroin and its metabolites: relevance to heroin use disorder
Abstract
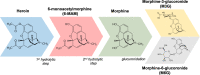
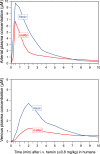
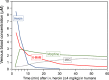
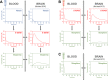
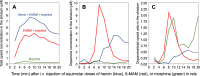
Heroin is an opioid agonist commonly abused for its rewarding effects. Since its synthesis at the end of the nineteenth century, its popularity as a recreational drug has ebbed and flowed. In the last three decades, heroin use has increased again, and yet the pharmacology of heroin is still poorly understood. After entering the body, heroin is rapidly deacetylated to 6-monoacetylmorphine (6-MAM), which is then deacetylated to morphine. Thus, drug addiction literature has long settled on the notion that heroin is little more than a pro-drug. In contrast to these former views, we will argue for a more complex interplay among heroin and its active metabolites: 6-MAM, morphine, and morphine-6-glucuronide (M6G). In particular, we propose that the complex temporal pattern of heroin effects results from the sequential, only partially overlapping, actions not only of 6-MAM, morphine, and M6G, but also of heroin per se, which, therefore, should not be seen as a mere brain-delivery system for its active metabolites. We will first review the literature concerning the pharmacokinetics and pharmacodynamics of heroin and its metabolites, then examine their neural and behavioral effects, and finally discuss the possible implications of these data for a better understanding of opioid reward and heroin addiction. By so doing we hope to highlight research topics to be investigated by future clinical and pre-clinical studies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
6-Monoacetylmorphine (6-MAM), Not Morphine, Is Responsible for the Rapid Neural Effects Induced by Intravenous Heroin.ACS Chem Neurosci. 2019 Aug 21;10(8):3409-3414. doi: 10.1021/acschemneuro.9b00305. Epub 2019 Jul 8. ACS Chem Neurosci. 2019. PMID: 31268284
-
P-glycoprotein (MDR1/ABCB1) Restricts Brain Penetration of the Main Active Heroin Metabolites 6-monoacetylmorphine (6-MAM) and Morphine in Mice.Pharm Res. 2023 Aug;40(8):1885-1899. doi: 10.1007/s11095-023-03545-6. Epub 2023 Jun 21. Pharm Res. 2023. PMID: 37344602
-
mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine.Biochem Pharmacol. 2001 Aug 15;62(4):447-55. doi: 10.1016/s0006-2952(01)00689-x. Biochem Pharmacol. 2001. PMID: 11448454
-
Metabolism and metabolomics of opiates: A long way of forensic implications to unravel.J Forensic Leg Med. 2019 Feb;61:128-140. doi: 10.1016/j.jflm.2018.12.005. Epub 2018 Dec 19. J Forensic Leg Med. 2019. PMID: 30621882 Review.
-
Morphine metabolites.Acta Anaesthesiol Scand. 1997 Jan;41(1 Pt 2):116-22. doi: 10.1111/j.1399-6576.1997.tb04625.x. Acta Anaesthesiol Scand. 1997. PMID: 9061094 Review.
Cited by
-
A Case of Ticagrelor-Induced Sinus Pause.Cureus. 2023 Jul 13;15(7):e41821. doi: 10.7759/cureus.41821. eCollection 2023 Jul. Cureus. 2023. PMID: 37575834 Free PMC article.
-
Glymphatic-System Function Is Associated with Addiction and Relapse in Heroin Dependents Undergoing Methadone Maintenance Treatment.Brain Sci. 2023 Sep 7;13(9):1292. doi: 10.3390/brainsci13091292. Brain Sci. 2023. PMID: 37759893 Free PMC article.
-
NEFL Modulates NRN1-Mediated Mitochondrial Pathway to Promote Diacetylmorphine-Induced Neuronal Apoptosis.Mol Neurobiol. 2024 Nov 19. doi: 10.1007/s12035-024-04629-z. Online ahead of print. Mol Neurobiol. 2024. PMID: 39557800
-
Photo-affinity and Metabolic Labeling Probes Based on the Opioid Alkaloids.Chembiochem. 2024 Mar 15;25(6):e202300841. doi: 10.1002/cbic.202300841. Epub 2024 Feb 19. Chembiochem. 2024. PMID: 38289703 Free PMC article.
-
Evaluation of heroin-assisted treatment in Norway: protocol for a mixed methods study.BMC Health Serv Res. 2024 Mar 29;24(1):398. doi: 10.1186/s12913-024-10767-w. BMC Health Serv Res. 2024. PMID: 38553691 Free PMC article.
References
-
- Merlin MD. On the trail of the ancient opium poppy: natural and early cultural history of papaver somniferum. Assoc Univ Press East Brunswick, New Jersey. 1984.
-
- Dikötter F, Laamann LP, Xun Z. (eds) Narcotic culture: a history of drugs in China (Hong Kong University Press, 2004).
-
- Courtright DT. (eds) Dark paradise: a history of opiate use in America (Harvard University Press, Cambridge, 1982).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

