Observational study of HR+/HER2- metastatic breast cancer patients treated with abemaciclib in Spain in the Named Patient Use Program (AbemusS)
- PMID: 37029241
- PMCID: PMC10462534
- DOI: 10.1007/s12094-023-03159-9
Observational study of HR+/HER2- metastatic breast cancer patients treated with abemaciclib in Spain in the Named Patient Use Program (AbemusS)
Abstract
Introduction/objectives: To describe abemaciclib use in patients with hormone receptor-positive, human epidermal growth factor receptor-negative (HR+/HER2-) metastatic breast cancer (mBC) who participated in the Named Patient Use program (NPU) in Spain.
Material and methods: This retrospective study was based on medical record review of patients across 20 centers during 2018/2019. Patients were followed up until death, enrolment in a clinical trial, loss of follow-up or study end. Clinical and demographic characteristics, treatment patterns and abemaciclib effectiveness were analyzed; time-to-event and median times were estimated using the Kaplan-Meier (KM) method.
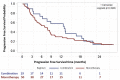
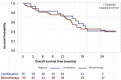
Results: The study included 69 female patients with mBC (mean age 60.4 ± 12.4 years), 86% of whom had an initial diagnosis of early BC and 20% had an ECOG ≥ 2. Median follow-up was 23 months (range 16-28). Metastases were frequently observed in bone (79%) and visceral tissue (65%), with 47% having metastases in > 2 sites. Median number of treatment lines before abemaciclib was 6 (range 1-10). Abemaciclib monotherapy was received by 72% of patients and combination therapy with endocrine therapy by 28% of patients; 54% of patients required dose adjustments, with a median time to first adjustment of 1.8 months. Abemaciclib was discontinued in 86% of patients after a median of 7.7 months (13.2 months for combination therapy and 7.0 months for monotherapy) mainly due to disease progression (69%).
Conclusion: These results suggest that abemaciclib is effective, as monotherapy and in combination, for patients with heavily pretreated mBC, consistent with clinical trial results.
Keywords: Abemaciclib; Effectiveness; HR+/HER2− Spain; Metastatic breast cancer; Real world.
© 2023. The Author(s).
Conflict of interest statement
MaA, SD, AM and JMC are employees and shareholders of Eli Lilly and Company. SB, JMGG, MiA, MAS and JG received an economic compensation from Eli Lilly and Co. for their participation in the study (consultancy and/or data collection tasks).
Figures

Similar articles
-
Abemaciclib for treating patients with HR+/HER2- metastatic breast cancer: a real-world study in France, Italy and Spain.Future Oncol. 2024;20(31):2371-2384. doi: 10.1080/14796694.2024.2368455. Epub 2024 Aug 8. Future Oncol. 2024. PMID: 39115881 Free PMC article.
-
nextMONARCH Phase 2 randomized clinical trial: overall survival analysis of abemaciclib monotherapy or in combination with tamoxifen in patients with endocrine-refractory HR + , HER2- metastatic breast cancer.Breast Cancer Res Treat. 2022 Aug;195(1):55-64. doi: 10.1007/s10549-022-06662-9. Epub 2022 Jul 12. Breast Cancer Res Treat. 2022. PMID: 35829935 Free PMC article. Clinical Trial.
-
Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial.Lancet Oncol. 2020 Jun;21(6):763-775. doi: 10.1016/S1470-2045(20)30112-1. Epub 2020 Apr 27. Lancet Oncol. 2020. PMID: 32353342 Clinical Trial.
-
Abemaciclib: A Review in Early Breast Cancer with a High Risk of Recurrence.Target Oncol. 2023 Mar;18(2):287-294. doi: 10.1007/s11523-023-00952-y. Epub 2023 Feb 24. Target Oncol. 2023. PMID: 36826463 Review.
-
Abemaciclib: The Newest CDK4/6 Inhibitor for the Treatment of Breast Cancer.Ann Pharmacother. 2019 Feb;53(2):178-185. doi: 10.1177/1060028018795146. Epub 2018 Aug 13. Ann Pharmacother. 2019. PMID: 30099886 Review.
Cited by
-
Abemaciclib for treating patients with HR+/HER2- metastatic breast cancer: a real-world study in France, Italy and Spain.Future Oncol. 2024;20(31):2371-2384. doi: 10.1080/14796694.2024.2368455. Epub 2024 Aug 8. Future Oncol. 2024. PMID: 39115881 Free PMC article.
-
Abemaciclib for the Treatment of HR+HER2- Metastatic Breast Cancer: An Institutional Experience.Cancers (Basel). 2024 May 10;16(10):1828. doi: 10.3390/cancers16101828. Cancers (Basel). 2024. PMID: 38791907 Free PMC article.
References
-
- Estimaciones de la incidencia del cáncer en España, 2022. Red Española de Registros de Cáncer (REDECAN) [Internet]. 2022. https://redecan.org/storage/documents/873877e1-af1b-43fe-8d97-0ee1434fe2.... Cited 12 Sept 2022.
-
- Grupo Español de Investigación en cáncer de mama (GEICAM). Guía GEICAM de Práctica Clínica Para el Diagnóstico y Tratamiento del Cáncer de Mama Metastásico GUÍA RESUMIDA 2015 [Internet]. San Sebastián de los Reyes; 2015. http://www.geicam.org. Cited 13 Nov 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

