Combinatorial gene therapy for epilepsy: Gene sequence positioning and AAV serotype influence expression and inhibitory effect on seizures
- PMID: 37029201
- PMCID: PMC10457185
- DOI: 10.1038/s41434-023-00399-w
Combinatorial gene therapy for epilepsy: Gene sequence positioning and AAV serotype influence expression and inhibitory effect on seizures
Abstract
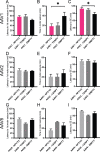
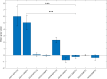
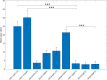
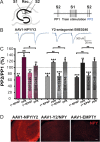
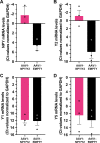
Gene therapy with AAV vectors carrying genes for neuropeptide Y and its receptor Y2 has been shown to inhibit seizures in multiple animal models of epilepsy. It is however unknown how the AAV serotype or the sequence order of these two transgenes in the expression cassette affects the actual parenchymal gene expression levels and the seizure-suppressant efficacy. To address these questions, we compared three viral vector serotypes (AAV1, AAV2 and AAV8) and two transgene sequence orders (NPY-IRES-Y2 and Y2-IRES-NPY) in a rat model of acutely induced seizures. Wistar male rats were injected bilaterally with viral vectors and 3 weeks later acute seizures were induced by a subcutaneous injection of kainate. The latency until 1st motor seizure, time spent in motor seizure and latency to status epilepticus were measured to evaluate the seizure-suppressing efficacy of these vectors compared to an empty cassette control vector. Based on the results, the effect of the AAV1-NPY-IRES-Y2 vector was further investigated by in vitro electrophysiology, and its ability to achieve transgene overexpression in resected human hippocampal tissue was evaluated. The AAV1-NPY-IRES-Y2 proved to be better to any other serotype or gene sequence considering both transgene expression and ability to suppress induced seizures in rats. The vector also demonstrated transgene-induced decrease of glutamate release from excitatory neuron terminals and significantly increased both NPY and Y2 expression in resected human hippocampal tissue from patients with drug-resistant temporal lobe epilepsy. These results validate the feasibility of NPY/Y2 receptor gene therapy as a therapeutic opportunity in focal epilepsies.
© 2023. The Author(s).
Conflict of interest statement
This study was sponsored by the spin-off company CombiGene AB. MK and DPDW are co-founders, shareowners, and consultants of CombiGene AB. Esbjörn Melin is employed and has shares in CombiGene AB.
Figures

Similar articles
-
Gene therapy mediated seizure suppression in Genetic Generalised Epilepsy: Neuropeptide Y overexpression in a rat model.Neurobiol Dis. 2018 May;113:23-32. doi: 10.1016/j.nbd.2018.01.016. Epub 2018 Feb 4. Neurobiol Dis. 2018. PMID: 29414380
-
Translational approach for gene therapy in epilepsy: Model system and unilateral overexpression of neuropeptide Y and Y2 receptors.Neurobiol Dis. 2016 Feb;86:52-61. doi: 10.1016/j.nbd.2015.11.014. Epub 2015 Dec 1. Neurobiol Dis. 2016. PMID: 26607785
-
Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus.J Neurosci. 2004 Mar 24;24(12):3051-9. doi: 10.1523/JNEUROSCI.4056-03.2004. J Neurosci. 2004. PMID: 15044544 Free PMC article.
-
Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy?Neuropeptides. 2004 Aug;38(4):245-52. doi: 10.1016/j.npep.2004.05.004. Neuropeptides. 2004. PMID: 15337376 Review.
-
Neuropeptide Y overexpression using recombinant adeno-associated viral vectors.Neurotherapeutics. 2009 Apr;6(2):300-6. doi: 10.1016/j.nurt.2009.01.012. Neurotherapeutics. 2009. PMID: 19332323 Free PMC article. Review.
Cited by
-
Recent advances and current status of gene therapy for epilepsy.World J Pediatr. 2024 Nov;20(11):1115-1137. doi: 10.1007/s12519-024-00843-w. Epub 2024 Oct 12. World J Pediatr. 2024. PMID: 39395088 Review.
-
Gene therapy for epilepsy targeting neuropeptide Y and its Y2 receptor to dentate gyrus granule cells.EMBO Rep. 2024 Oct;25(10):4387-4409. doi: 10.1038/s44319-024-00244-0. Epub 2024 Sep 9. EMBO Rep. 2024. PMID: 39251828 Free PMC article.
-
Gene therapy for CNS disorders: modalities, delivery and translational challenges.Nat Rev Neurosci. 2024 Aug;25(8):553-572. doi: 10.1038/s41583-024-00829-7. Epub 2024 Jun 19. Nat Rev Neurosci. 2024. PMID: 38898231 Review.
-
Viral Vector-Based Gene Therapy for Epilepsy: What Does the Future Hold?Mol Diagn Ther. 2024 Jan;28(1):5-13. doi: 10.1007/s40291-023-00687-6. Epub 2023 Dec 16. Mol Diagn Ther. 2024. PMID: 38103141 Free PMC article.
References
-
- Yilmazer-Hanke DM, Wolf HK, Schramm J, Elger CE, Wiestler OD, Blümcke I. Subregional pathology of the amygdala complex and entorhinal region in surgical specimens from patients with pharmacoresistant temporal lobe epilepsy. J Neuropathol Exp Neurol. 2000;59:907–20. doi: 10.1093/jnen/59.10.907. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

