Hepatitis C and hepatitis B virus infection in hemodialysis patients after nationwide direct antiviral agents therapy-experience of 10 Romanian HD centers
- PMID: 37024632
- PMCID: PMC10560143
- DOI: 10.1007/s11255-023-03587-0
Hepatitis C and hepatitis B virus infection in hemodialysis patients after nationwide direct antiviral agents therapy-experience of 10 Romanian HD centers
Abstract
Purpose: End-stage kidney disease patients (ESKD) receiving hemodialysis (HD) are at a greater risk of hepatitis virus (HV) infections due to the invasive nature of the procedures, frequent hospital stays and surgeries, as well as the immune deficiency status of ESKD.
The aim: This study was to reassess the hepatitis virus infections prevalence in the HD population in Romania after 5 years of oral DAAs therapy and assess the impact on HD patients' outcomes in two cohorts (2015 and 2019).
Methods: We compared ESKD patients treated with HD in 10 HD centers from the historical regions of Romania in 2015 (n = 1401, Mean age 59.7 ± 12.92 years) with patients treated in the same centers in 2019 (n = 1698, mean age 61 ± 12.93 years). All patients went through HD therapy for more than 90 days.
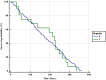
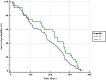
Results: The patients from the 2019 cohort were significantly older (p = 0.005), had a longer duration of HD therapy (p < 0.0001), and had more vascular calcifications (p = 0.015); the crude one-year mortality rate did not differ from the 2015 cohort (9.9 vs. 10.7%, p = 0.46). The prevalence of HBV infection did not differ between the cohorts (4.7% vs. 4.8, p = 0.604) but the prevalence of HCV significantly decreased from 2015 to 2019 (16.9 vs. 10.5%, p < 0.0001).
Conclusion: After 15 years of a nationwide infection prevention program for HV infections and 5 years of DAAs treatment in Romania, the prevalence of HBV did not change but HCV infections decreased significantly, however, it still remained high.
Keywords: DAA; ESKD; Hemodialysis; Hepatitis B virus; Hepatitis C virus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hepatitis B and C virus infection in the hemodialysis population from three romanian regions.Nephron. 2015;129(3):202-8. doi: 10.1159/000371450. Epub 2015 Mar 4. Nephron. 2015. PMID: 25765861
-
Prevention of hepatitis B virus and hepatitis C virus transmission in hemodialysis centers: review of current international recommendations.Arab J Nephrol Transplant. 2011 Jan;4(1):35-47. doi: 10.4314/ajnt.v4i1.63154. Arab J Nephrol Transplant. 2011. PMID: 21469594 Review.
-
Prevalence of occult hepatitis B and hepatitis C virus infections in Turkish hemodialysis patients.Ren Fail. 2006;28(8):729-35. doi: 10.1080/08860220600925602. Ren Fail. 2006. PMID: 17162434
-
A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania.J Gastrointestin Liver Dis. 2010 Mar;19(1):43-8. doi: 10.1007/s11749-009-0177-3. J Gastrointestin Liver Dis. 2010. PMID: 20361074
-
Global epidemiology of HBV infection among hemodialysis patients: A systematic review and meta-analysis.Microb Pathog. 2023 Jun;179:106080. doi: 10.1016/j.micpath.2023.106080. Epub 2023 Mar 21. Microb Pathog. 2023. PMID: 36948364 Review.
References
-
- European centre for disease prevention and control. Systematic review on hepatitis B and C prevalence in the EU/EEA 2016 TQ-02-16-837-EN-N
-
- Vladutiu DS, Cosa A, Neamtu A, State D, Braila M, Gherman M, Patiu IM, Dulau-Florea I. Infections with hepatitis B and C viruses in patients on maintenance dialysis in Romania and in former communist countries: yellow spots on a blank map? J Viral Hepat. 2000;7:313–319. doi: 10.1046/j.1365-2893.2000.00222.x. - DOI - PubMed
-
- Romanian ministry of health-ORDER Nr. 1718 December 23, 2004 on the approval of the regulation on the organization and functioning of dialysis units public and private
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical