Reversible protein assemblies in the proteostasis network in health and disease
- PMID: 37021114
- PMCID: PMC10067754
- DOI: 10.3389/fmolb.2023.1155521
Reversible protein assemblies in the proteostasis network in health and disease
Abstract
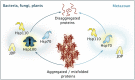
While proteins populating their native conformations constitute the functional entities of cells, protein aggregates are traditionally associated with cellular dysfunction, stress and disease. During recent years, it has become clear that large aggregate-like protein condensates formed via liquid-liquid phase separation age into more solid aggregate-like particles that harbor misfolded proteins and are decorated by protein quality control factors. The constituent proteins of the condensates/aggregates are disentangled by protein disaggregation systems mainly based on Hsp70 and AAA ATPase Hsp100 chaperones prior to their handover to refolding and degradation systems. Here, we discuss the functional roles that condensate formation/aggregation and disaggregation play in protein quality control to maintain proteostasis and why it matters for understanding health and disease.
Keywords: Hsp100; Hsp70; aggregate; biomolecular condensate; degradation; disaggregation; phase separation; refolding.
Copyright © 2023 Kohler and Andréasson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Crosstalk between Biomolecular Condensates and Proteostasis.Cells. 2022 Aug 4;11(15):2415. doi: 10.3390/cells11152415. Cells. 2022. PMID: 35954258 Free PMC article. Review.
-
Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation.Front Mol Biosci. 2015 May 19;2:22. doi: 10.3389/fmolb.2015.00022. eCollection 2015. Front Mol Biosci. 2015. PMID: 26042222 Free PMC article. Review.
-
Protein Disaggregation in Multicellular Organisms.Trends Biochem Sci. 2018 Apr;43(4):285-300. doi: 10.1016/j.tibs.2018.02.003. Epub 2018 Feb 28. Trends Biochem Sci. 2018. PMID: 29501325 Review.
-
The Diverse Functions of Small Heat Shock Proteins in the Proteostasis Network.J Mol Biol. 2022 Jan 15;434(1):167157. doi: 10.1016/j.jmb.2021.167157. Epub 2021 Jul 14. J Mol Biol. 2022. PMID: 34271010 Review.
-
Solid-to-liquid phase transition in the dissolution of cytosolic misfolded-protein aggregates.iScience. 2023 Oct 28;26(12):108334. doi: 10.1016/j.isci.2023.108334. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025775 Free PMC article.
Cited by
-
Biophysical Characterization of the Binding Mechanism between the MATH Domain of SPOP and Its Physiological Partners.Int J Mol Sci. 2023 Jun 14;24(12):10138. doi: 10.3390/ijms241210138. Int J Mol Sci. 2023. PMID: 37373284 Free PMC article.
-
Better Together: Interorganellar Communication in the Regulation of Proteostasis.Contact (Thousand Oaks). 2024 Oct 8;7:25152564241272245. doi: 10.1177/25152564241272245. eCollection 2024 Jan-Dec. Contact (Thousand Oaks). 2024. PMID: 39385949 Free PMC article. Review.
-
Nuclear Hsp104 safeguards the dormant translation machinery during quiescence.Nat Commun. 2024 Jan 5;15(1):315. doi: 10.1038/s41467-023-44538-8. Nat Commun. 2024. PMID: 38182580 Free PMC article.
-
Protein aggregation and biomolecular condensation in hypoxic environments (Review).Int J Mol Med. 2024 Apr;53(4):33. doi: 10.3892/ijmm.2024.5357. Epub 2024 Feb 16. Int J Mol Med. 2024. PMID: 38362920 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

