Phase separation in cancer at a glance
- PMID: 37005672
- PMCID: PMC10067312
- DOI: 10.1186/s12967-023-04082-x
Phase separation in cancer at a glance
Abstract
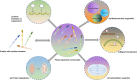
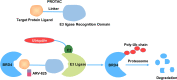
Eukaryotic cells are segmented into multiple compartments or organelles within the cell that regulate distinct chemical and biological processes. Membrane-less organelles are membrane-less microscopic cellular compartments that contain protein and RNA molecules that perform a wide range of functions. Liquid-liquid phase separation (LLPS) can reveal how membrane-less organelles develop via dynamic biomolecule assembly. LLPS either segregates undesirable molecules from cells or aggregates desired ones in cells. Aberrant LLPS results in the production of abnormal biomolecular condensates (BMCs), which can cause cancer. Here, we explore the intricate mechanisms behind the formation of BMCs and its biophysical properties. Additionally, we discuss recent discoveries related to biological LLPS in tumorigenesis, including aberrant signaling and transduction, stress granule formation, evading growth arrest, and genomic instability. We also discuss the therapeutic implications of LLPS in cancer. Understanding the concept and mechanism of LLPS and its role in tumorigenesis is crucial for antitumor therapeutic strategies.
Keywords: Cancer; Cancer biology; Mechanism; Phase separation; Therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflict of interest.
Figures

Similar articles
-
Emerging Implications of Phase Separation in Cancer.Adv Sci (Weinh). 2022 Nov;9(31):e2202855. doi: 10.1002/advs.202202855. Epub 2022 Sep 18. Adv Sci (Weinh). 2022. PMID: 36117111 Free PMC article. Review.
-
Aberrant phase separation and cancer.FEBS J. 2022 Jan;289(1):17-39. doi: 10.1111/febs.15765. Epub 2021 Mar 3. FEBS J. 2022. PMID: 33583140 Review.
-
Role of liquid-liquid phase separation in cancer: Mechanisms and therapeutic implications.Cancer Innov. 2024 Sep 17;3(5):e144. doi: 10.1002/cai2.144. eCollection 2024 Oct. Cancer Innov. 2024. PMID: 39290787 Free PMC article. Review.
-
Liquid-liquid phase separation in biology: mechanisms, physiological functions and human diseases.Sci China Life Sci. 2020 Jul;63(7):953-985. doi: 10.1007/s11427-020-1702-x. Epub 2020 Apr 30. Sci China Life Sci. 2020. PMID: 32548680 Review.
-
Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications.J Biol Chem. 2019 Oct 4;294(40):14823-14835. doi: 10.1074/jbc.REV119.007895. Epub 2019 Aug 23. J Biol Chem. 2019. PMID: 31444270 Free PMC article. Review.
Cited by
-
Advancements in melanoma immunotherapy: the emergence of Extracellular Vesicle Vaccines.Cell Death Discov. 2024 Aug 23;10(1):374. doi: 10.1038/s41420-024-02150-9. Cell Death Discov. 2024. PMID: 39174509 Free PMC article. Review.
-
Subcellular Drug Distribution: Exploring Organelle-Specific Characteristics for Enhanced Therapeutic Efficacy.Pharmaceutics. 2024 Sep 4;16(9):1167. doi: 10.3390/pharmaceutics16091167. Pharmaceutics. 2024. PMID: 39339204 Free PMC article. Review.
-
EphA2 as a phase separation protein associated with ferroptosis and immune cell infiltration in colorectal cancer.Aging (Albany NY). 2023 Nov 16;15(22):12952-12965. doi: 10.18632/aging.205212. Epub 2023 Nov 16. Aging (Albany NY). 2023. PMID: 37980165 Free PMC article.
-
Phase separation is regulated by post-translational modifications and participates in the developments of human diseases.Heliyon. 2024 Jul 3;10(13):e34035. doi: 10.1016/j.heliyon.2024.e34035. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39071719 Free PMC article. Review.
-
Influence of HIV-1 Genomic RNA on the Formation of Gag Biomolecular Condensates.J Mol Biol. 2023 Aug 15;435(16):168190. doi: 10.1016/j.jmb.2023.168190. Epub 2023 Jun 27. J Mol Biol. 2023. PMID: 37385580 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

