Inflammation-Associated Lung Tissue Remodeling and Fibrosis in Morphine-Dependent SIV-Infected Macaques
- PMID: 37003622
- PMCID: PMC10116601
- DOI: 10.1016/j.ajpath.2022.12.016
Inflammation-Associated Lung Tissue Remodeling and Fibrosis in Morphine-Dependent SIV-Infected Macaques
Abstract
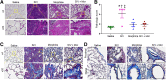
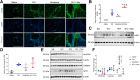
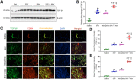
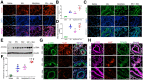
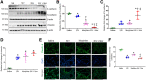
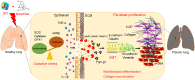
With the advent of antiretroviral therapy, improved survival of people with HIV (PWH) is accompanied with increased prevalence of HIV-associated comorbidities. Chronic lung anomalies are recognized as one of the most devastating sequelae in PWH. The limited available data describing the lung complications in PWH with a history of opioid abuse warrants more research to better define the course of disease pathogenesis. The current study was conducted to investigate the progression of lung tissue remodeling in a morphine (Mor)-exposed rhesus macaque model of SIV infection. Pathologic features of lung remodeling, including histopathologic changes, oxidative stress, inflammation, and proliferation of fibroblasts, were investigated in archival lung tissues of SIVmac-251/macaque model with or without Mor dependence. Lungs of Mor-exposed, SIV-infected macaques exhibited significant fibrotic changes and collagen deposition in the alveolar and the bronchiolar region. There was increased oxidative stress, profibrotic transforming growth factor-β, fibroblast proliferation and trans-differentiation, epithelial-mesenchymal transition, and matrix degradation in SIV-infected macaques, which was further exacerbated in the lungs of Mor-exposed macaques. Interestingly, there was decreased inflammation-associated remodeling in Mor-dependent SIV-infected macaques compared with SIV-infected macaques that did not receive Mor. Thus, the current findings suggest that SIV independently induces fibrotic changes in macaque lungs, which is further aggravated by Mor.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Elevated Inflammation Associated with Markers of Neutrophil Function and Gastrointestinal Disruption in Pilot Study of Plasmodium fragile Co-Infection of ART-Treated SIVmac239+ Rhesus Macaques.Viruses. 2024 Jun 27;16(7):1036. doi: 10.3390/v16071036. Viruses. 2024. PMID: 39066199 Free PMC article.
-
Neuroinflammation in the Dorsal Root Ganglia and Dorsal Horn Contributes to Persistence of Nociceptor Sensitization in SIV-Infected Antiretroviral Therapy-Treated Macaques.Am J Pathol. 2023 Dec;193(12):2017-2030. doi: 10.1016/j.ajpath.2023.08.014. Epub 2023 Sep 19. Am J Pathol. 2023. PMID: 37734588 Free PMC article.
-
System-wide identification of myeloid markers of TB disease and HIV-induced reactivation in the macaque model of Mtb infection and Mtb/SIV co-infection.Front Immunol. 2022 Oct 5;13:777733. doi: 10.3389/fimmu.2022.777733. eCollection 2022. Front Immunol. 2022. PMID: 36275677 Free PMC article.
-
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD006829. doi: 10.1002/14651858.CD006829.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972099 Free PMC article. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Molecular mechanism of hypoxia and alpha-ketoglutaric acid on collagen expression in scleral fibroblasts.Int J Ophthalmol. 2024 Oct 18;17(10):1780-1790. doi: 10.18240/ijo.2024.10.03. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430015 Free PMC article.
References
-
- Wallace J.M., Hansen N.I., Lavange L., Glassroth J., Browdy B.L., Rosen M.J., Kvale P.A., Mangura B.T., Reichman L.B., Hopewell P.C., Pulmonary Complications of HIV Infection Study Group Respiratory disease trends in the Pulmonary Complications of HIV Infection Study cohort. Am J Respir Crit Care Med. 1997;155:72–80. - PubMed
-
- Crothers K., Butt A.A., Gibert C.L., Rodriguez-Barradas M.C., Crystal S., Justice A.C., Veterans Aging Cohort 5 Project Team Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. - PubMed
-
- ten Freyhaus H., Vogel D., Lehmann C., Kummerle T., Wyen C., Fatkenheuer G., Rosenkranz S. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection. 2014;42:737–741. - PubMed
-
- Sabin C.A., Kunisaki K.M., Bagkeris E., Post F.A., Sachikonye M., Boffito M., Anderson J., Mallon P., Williams I., Vera J.H., Johnson M., Babalis D., Winston A. Respiratory symptoms and chronic bronchitis in people with and without HIV infection. HIV Med. 2021;22:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

