Capturing effects of blood flow on the transplanted decellularized nephron with intravital microscopy
- PMID: 37002341
- PMCID: PMC10066218
- DOI: 10.1038/s41598-023-31747-w
Capturing effects of blood flow on the transplanted decellularized nephron with intravital microscopy
Abstract
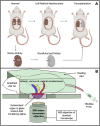
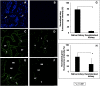
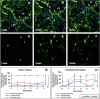
Organ decellularization creates cell-free, collagen-based extracellular matrices that can be used as scaffolds for tissue engineering applications. This technique has recently gained much attention, yet adequate scaffold repopulation and implantation remain a challenge. Specifically, there still needs to be a greater understanding of scaffold responses post-transplantation and ways we can improve scaffold durability to withstand the in vivo environment. Recent studies have outlined vascular events that limit organ decellularization/recellularization scaffold viability for long-term transplantation. However, these insights have relied on in vitro/in vivo approaches that need enhanced spatial and temporal resolutions to investigate such issues at the microvascular level. This study uses intravital microscopy to gain instant feedback on their structure, function, and deformation dynamics. Thus, the objective of this study was to capture the effects of in vivo blood flow on the decellularized glomerulus, peritubular capillaries, and tubules after autologous and allogeneic orthotopic transplantation into rats. Large molecular weight dextran molecules labeled the vasculature. They revealed substantial degrees of translocation from glomerular and peritubular capillary tracks to the decellularized tubular epithelium and lumen as early as 12 h after transplantation, providing real-time evidence of the increases in microvascular permeability. Macromolecular extravasation persisted for a week, during which the decellularized microarchitecture was significantly and comparably compromised and thrombosed in both autologous and allogeneic approaches. These results indicate that in vivo multiphoton microscopy is a powerful approach for studying scaffold viability and identifying ways to promote scaffold longevity and vasculogenesis in bioartificial organs.
© 2023. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures



Similar articles
-
Intravital microscopy datasets examining key nephron segments of transplanted decellularized kidneys.Sci Data. 2022 Sep 10;9(1):561. doi: 10.1038/s41597-022-01685-9. Sci Data. 2022. PMID: 36088356 Free PMC article.
-
Building a Total Bioartificial Heart: Harnessing Nature to Overcome the Current Hurdles.Artif Organs. 2018 Oct;42(10):970-982. doi: 10.1111/aor.13336. Epub 2018 Oct 16. Artif Organs. 2018. PMID: 30044011
-
Incompletely Decellularized Tracheal Matrix Scaffold for Tissue Engineering.Plast Reconstr Surg. 2024 May 1;153(5):932e-941e. doi: 10.1097/PRS.0000000000010771. Epub 2023 May 31. Plast Reconstr Surg. 2024. PMID: 37285021
-
Incorporation of nanoparticles into transplantable decellularized matrices: Applications and challenges.Int J Artif Organs. 2018 Aug;41(8):421-430. doi: 10.1177/0391398818775522. Epub 2018 May 28. Int J Artif Organs. 2018. PMID: 29807488 Review.
-
Organ reconstruction: Dream or reality for the future.Biomed Mater Eng. 2017;28(s1):S121-S127. doi: 10.3233/BME-171633. Biomed Mater Eng. 2017. PMID: 28372287 Review.
Cited by
-
Gray level co-occurrence matrix and wavelet analyses reveal discrete changes in proximal tubule cell nuclei after mild acute kidney injury.Sci Rep. 2023 Mar 10;13(1):4025. doi: 10.1038/s41598-023-31205-7. Sci Rep. 2023. PMID: 36899130 Free PMC article.
-
Computational approaches for evaluating morphological changes in the corneal stroma associated with decellularization.Front Bioeng Biotechnol. 2023 May 26;11:1105377. doi: 10.3389/fbioe.2023.1105377. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37304146 Free PMC article.
-
Integrated environmental and health economic assessments of novel xeno-keratografts addressing a growing public health crisis.Sci Rep. 2024 Oct 27;14(1):25600. doi: 10.1038/s41598-024-77783-y. Sci Rep. 2024. PMID: 39465317 Free PMC article.
-
A proposed model of xeno-keratoplasty using 3D printing and decellularization.Front Pharmacol. 2023 Sep 20;14:1193606. doi: 10.3389/fphar.2023.1193606. eCollection 2023. Front Pharmacol. 2023. PMID: 37799970 Free PMC article.
-
Enhancing the expression of a key mitochondrial enzyme at the inception of ischemia-reperfusion injury can boost recovery and halt the progression of acute kidney injury.Front Physiol. 2023 Feb 8;14:1024238. doi: 10.3389/fphys.2023.1024238. eCollection 2023. Front Physiol. 2023. PMID: 36846323 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

