FBXW7 attenuates tumor drug resistance and enhances the efficacy of immunotherapy
- PMID: 36998461
- PMCID: PMC10043335
- DOI: 10.3389/fonc.2023.1147239
FBXW7 attenuates tumor drug resistance and enhances the efficacy of immunotherapy
Abstract
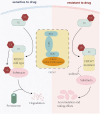
FBXW7 (F-box and WD repeat domain containing 7) is a critical subunit of the Skp1-Cullin1-F-box protein (SCF), acting as an E3 ubiquitin ligase by ubiquitinating targeted protein. Through degradation of its substrates, FBXW7 plays a pivotal role in drug resistance in tumor cells and shows the potential to rescue the sensitivity of cancer cells to drug treatment. This explains why patients with higher FBXW7 levels exhibit higher survival times and more favorable prognosis. Furthermore, FBXW7 has been demonstrated to enhance the efficacy of immunotherapy by targeting the degradation of specific proteins, as compared to the inactivated form of FBXW7. Additionally, other F-box proteins have also shown the ability to conquer drug resistance in certain cancers. Overall, this review aims to explore the function of FBXW7 and its specific effects on drug resistance in cancer cells.
Keywords: E3 ligase; FBXW7; cancer; drug resistance; immunotherapy.
Copyright © 2023 Chen, Lin, Zhao, Lin, Liu, Wang, Mui and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Molecular insights and clinical implications for the tumor suppressor role of SCFFBXW7 E3 ubiquitin ligase.Biochim Biophys Acta Rev Cancer. 2024 Sep;1879(5):189140. doi: 10.1016/j.bbcan.2024.189140. Epub 2024 Jun 21. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38909632 Review.
-
Recent Insight on Regulations of FBXW7 and Its Role in Immunotherapy.Front Oncol. 2022 Jun 24;12:925041. doi: 10.3389/fonc.2022.925041. eCollection 2022. Front Oncol. 2022. PMID: 35814468 Free PMC article. Review.
-
The Highs and Lows of FBXW7: New Insights into Substrate Affinity in Disease and Development.Cells. 2023 Aug 24;12(17):2141. doi: 10.3390/cells12172141. Cells. 2023. PMID: 37681873 Free PMC article. Review.
-
The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues.Cancer Rep (Hoboken). 2021 Aug;4(4):e1369. doi: 10.1002/cnr2.1369. Epub 2021 Apr 6. Cancer Rep (Hoboken). 2021. PMID: 33822486 Free PMC article. Review.
-
The SCF-type E3 Ubiquitin Ligases as Cancer Targets.Curr Cancer Drug Targets. 2016;16(2):119-29. doi: 10.2174/1568009616666151112122231. Curr Cancer Drug Targets. 2016. PMID: 26560120 Review.
Cited by
-
FBXW7 and human tumors: mechanisms of drug resistance and potential therapeutic strategies.Front Pharmacol. 2023 Nov 13;14:1278056. doi: 10.3389/fphar.2023.1278056. eCollection 2023. Front Pharmacol. 2023. PMID: 38027013 Free PMC article. Review.
-
Case report: Immunotherapy guided by molecular profiling of tumors: illustrative cases and literature review.Front Med (Lausanne). 2024 Jul 9;11:1403056. doi: 10.3389/fmed.2024.1403056. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39045411 Free PMC article.
-
5-Aza-2'-Deoxycytidine Alters the Methylation Profile of Bortezomib-Resistant U266 Multiple Myeloma Cells and Affects Their Proliferative Potential.Int J Mol Sci. 2023 Nov 26;24(23):16780. doi: 10.3390/ijms242316780. Int J Mol Sci. 2023. PMID: 38069103 Free PMC article.
-
MYCN in human development and diseases.Front Oncol. 2024 May 31;14:1417607. doi: 10.3389/fonc.2024.1417607. eCollection 2024. Front Oncol. 2024. PMID: 38884091 Free PMC article. Review.
References
-
- Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, et al. . Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res (2020) 26(11):2654–63. doi: 10.1158/1078-0432.CCR-19-3563 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

