Validation of the severe COVID-19 prognostic value of serum IL-6, IFN-λ3, CCL17, and calprotectin considering the timing of clinical need for prediction
- PMID: 36996138
- PMCID: PMC10062661
- DOI: 10.1371/journal.pone.0279897
Validation of the severe COVID-19 prognostic value of serum IL-6, IFN-λ3, CCL17, and calprotectin considering the timing of clinical need for prediction
Abstract
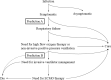
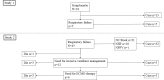
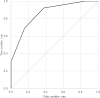
Although biomarkers to predict coronavirus disease 2019 (COVID-19) severity have been studied since the early pandemic, no clear guidelines on using them in clinical practice are available. Here, we examined the ability of four biomarkers to predict disease severity using conserved sera from COVID-19 patients who received inpatient care between January 1, 2020 and September 21, 2021 at the National Center for Global Health and Medicine, collected at the appropriate time for prediction. We predicted illness severity in two situations: 1) prediction of future oxygen administration for patients without oxygen support within 8 days of onset (Study 1) and 2) prediction of future mechanical ventilation support (excluding non-invasive positive pressure ventilation) or death of patients within 4 days of the start of oxygen administration (Study 2). Interleukin-6, IFN-λ3, thymus and activation-regulated chemokine, and calprotectin were measured retrospectively. Other laboratory and clinical information were collected from medical records. AUCs were calculated from ROC curves and compared for the predictive ability of the four biomarkers. Study 1 included 18 patients, five of whom had developed oxygen needs. Study 2 included 45 patients, 13 of whom required ventilator management or died. In Study 1, IFN-λ3 showed a good predictive ability with an AUC of 0.92 (95% CI 0.76-1.00). In Study 2, the AUC of each biomarker was 0.70-0.74. The number of biomarkers above the cutoff showed the possibility of good prediction with an AUC of 0.86 (95% CI 0.75-0.97). When two or more biomarkers were positive, sensitivity and specificity were 0.92 and 0.63, respectively. In terms of biomarker testing at times when prognostication may be clinically useful, IFN-λ3 was predictive of oxygenation demand and a combination of the four biomarkers was predictive of mechanical ventilator requirement.
Copyright: © 2023 Yamamoto et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
K.Y. received research grants from Sanyo Chemical Industries, Ltd. [no grant number] for the submitted work. S. N. and M. Kurokawa. are employees of Sanyo Chemical Industries, Ltd. K.Y. received research grants from Fujirebio, Inc., Mizuho Medy, Co., Ltd., VisGene, Co., Ltd., Canon medical systems Co., and CarbGeM Inc., and M. Kimura received research grants from SB Coronavirus Inspection Center Corp., Canon medical systems Co., and Ezaki Glico Co., Ltd., and W. S received research grants from SB Coronavirus Inspection Center Corp. and Nippon genetics Co., Ltd. outside the submitted work. This does not alter our adherence to PLOS ONE policies on sharing data and materials. K. Y. and Sanyo Chemical Industries have a patent pending on the calprotectin assay. There are no additional patents, products in development or marketed products associated with this research to declare.
Figures

Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Serum CCL17 level becomes a predictive marker to distinguish between mild/moderate and severe/critical disease in patients with COVID-19.Gene. 2021 Jan 15;766:145145. doi: 10.1016/j.gene.2020.145145. Epub 2020 Sep 14. Gene. 2021. PMID: 32941953 Free PMC article.
-
IFN-λ3 and CCL17 as predictors of disease progression in patients with mild to moderate COVID-19: A cohort study in a real-world setting.Respir Investig. 2023 Mar;61(2):153-156. doi: 10.1016/j.resinv.2022.12.006. Epub 2023 Jan 12. Respir Investig. 2023. PMID: 36682084 Free PMC article.
-
Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis.Clin Chem Lab Med. 2020 Jun 25;58(7):1021-1028. doi: 10.1515/cclm-2020-0369. Clin Chem Lab Med. 2020. PMID: 32286245 Review.
-
Potential role of biochemical markers in the prognosis of COVID-19 patients.SAGE Open Med. 2022 Jul 5;10:20503121221108613. doi: 10.1177/20503121221108613. eCollection 2022. SAGE Open Med. 2022. PMID: 35832258 Free PMC article. Review.
References
-
- Sayah W, Berkane I, Guermache I, Sabri M, Lakhal FZ, Yasmine Rahali S, et al.. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine. 2021. May;141: 155428. doi: 10.1016/j.cyto.2021.155428 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

