Constitutive and conditional gene knockout mice for the study of intervertebral disc degeneration: Current status, decision considerations, and future possibilities
- PMID: 36994464
- PMCID: PMC10041386
- DOI: 10.1002/jsp2.1242
Constitutive and conditional gene knockout mice for the study of intervertebral disc degeneration: Current status, decision considerations, and future possibilities
Abstract
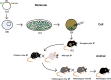
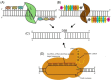
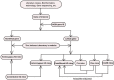
There have been an increasing number of patients with degenerative disc diseases due to the aging population. In light of this, studies on the pathogenesis of intervertebral disc degeneration have become a hot topic, and gene knockout mice have become a valuable tool in this field of research. With the development of science and technology, constitutive gene knockout mice can be constructed using homologous recombination, zinc finger nuclease, transcription activator-like effector nuclease technology and clustered regularly interspaced short palindromic repeats/Cas9 (CRISPR/Cas9) system, and conditional gene knockout mice can be constructed using the Cre/LoxP system. The gene-edited mice using these techniques have been widely used in the studies on disc degeneration. This paper reviews the development process and principles of these technologies, functions of the edited genes in disc degeneration, advantages, and disadvantages of different methods and possible targets of the specific Cre recombinase in intervertebral discs. Recommendations for the choice of suitable gene-edited model mice are presented. At the same time, possible technological improvements in the future are also discussed.
Keywords: construction; gene edition technology; gene targeting; guideline; intervertebral disc degeneration; knockout; mouse model; specific Cre recombinase.
© 2023 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Gene targeting technologies in rats: zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats.Dev Growth Differ. 2014 Jan;56(1):46-52. doi: 10.1111/dgd.12110. Epub 2013 Dec 27. Dev Growth Differ. 2014. PMID: 24372523 Review.
-
Advance of Clustered Regularly Interspaced Short Palindromic Repeats-Cas9 System and Its Application in Crop Improvement.Front Plant Sci. 2022 May 12;13:839001. doi: 10.3389/fpls.2022.839001. eCollection 2022. Front Plant Sci. 2022. PMID: 35645999 Free PMC article. Review.
-
Building Cre Knockin Rat Lines Using CRISPR/Cas9.Methods Mol Biol. 2017;1642:37-52. doi: 10.1007/978-1-4939-7169-5_3. Methods Mol Biol. 2017. PMID: 28815492
-
Gene Editing in Clinical Practice: Where are We?Indian J Clin Biochem. 2019 Jan;34(1):19-25. doi: 10.1007/s12291-018-0804-4. Epub 2019 Jan 1. Indian J Clin Biochem. 2019. PMID: 30728669 Free PMC article. Review.
-
Can genetic engineering-based methods for gene function identification be eclipsed by genome editing in plants? A comparison of methodologies.Mol Genet Genomics. 2021 May;296(3):485-500. doi: 10.1007/s00438-021-01769-y. Epub 2021 Mar 9. Mol Genet Genomics. 2021. PMID: 33751237 Review.
Cited by
-
Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus.J Dev Biol. 2024 Jul 3;12(3):18. doi: 10.3390/jdb12030018. J Dev Biol. 2024. PMID: 39051200 Free PMC article. Review.
-
Gasdermin D promotes development of intestinal tumors through regulating IL-1β release and gut microbiota composition.Cell Commun Signal. 2024 Oct 21;22(1):511. doi: 10.1186/s12964-024-01890-6. Cell Commun Signal. 2024. PMID: 39434144 Free PMC article.
References
-
- Xi Y, Ma J, Chen YJC. PTEN promotes intervertebral disc degeneration by regulating nucleus pulposus cell behaviors. Cell Biol Int. 2020;44(2):583‐592. - PubMed
-
- Sampara P, Banala R, Vemuri S, Av G, Gpv SJG. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25(2):67‐82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

