Protein Degradation by Gammaherpesvirus RTAs: More Than Just Viral Transactivators
- PMID: 36992439
- PMCID: PMC10055789
- DOI: 10.3390/v15030730
Protein Degradation by Gammaherpesvirus RTAs: More Than Just Viral Transactivators
Abstract
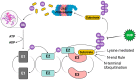
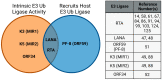
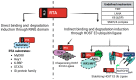
Kaposi's sarcoma-associated herpesvirus (KSHV) is a member of the Gammaherpesvirus subfamily that encodes several viral proteins with intrinsic E3 ubiquitin ligase activity or the ability to hijack host E3 ubiquitin ligases to modulate the host's immune response and to support the viral life cycle. This review focuses specifically on how the immediate-early KSHV protein RTA (replication and transcription activator) hijacks the host's ubiquitin-proteasome pathway (UPP) to target cellular and viral factors for protein degradation to allow for robust lytic reactivation. Notably, RTA's targets are either potent transcription repressors or they are activators of the innate and adaptive immune response, which block the lytic cycle of the virus. This review mainly focuses on what is currently known about the role of the E3 ubiquitin ligase activity of KSHV RTA in the regulation of the KSHV life cycle, but we will also discuss the potential role of other gammaherpesviral RTA homologs in UPP-mediated protein degradation.
Keywords: E3 ubiquitin ligases; KSHV; RTA; gammaherpesviruses; protein degradation; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
KSHV RTA Induces Degradation of the Host Transcription Repressor ID2 To Promote the Viral Lytic Cycle.J Virol. 2022 Jun 22;96(12):e0010122. doi: 10.1128/jvi.00101-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604218 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus K-Rta exhibits SUMO-targeting ubiquitin ligase (STUbL) like activity and is essential for viral reactivation.PLoS Pathog. 2013;9(8):e1003506. doi: 10.1371/journal.ppat.1003506. Epub 2013 Aug 22. PLoS Pathog. 2013. PMID: 23990779 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus processivity factor (PF-8) recruits cellular E3 ubiquitin ligase CHFR to promote PARP1 degradation and lytic replication.PLoS Pathog. 2021 Jan 28;17(1):e1009261. doi: 10.1371/journal.ppat.1009261. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33508027 Free PMC article.
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
-
KSHV reactivation and novel implications of protein isomerization on lytic switch control.Viruses. 2015 Jan 12;7(1):72-109. doi: 10.3390/v7010072. Viruses. 2015. PMID: 25588053 Free PMC article. Review.
Cited by
-
Conserved linear motif within the immediate early protein ORF45 promotes its engagement with KSHV lytic cycle-promoting forkhead transcription factors, FOXK1 and FOXK2.J Virol. 2024 Oct 22;98(10):e0088624. doi: 10.1128/jvi.00886-24. Epub 2024 Sep 17. J Virol. 2024. PMID: 39287387
-
E3 Ubiquitin Ligases in Gammaherpesviruses and HIV: A Review of Virus Adaptation and Exploitation.Viruses. 2023 Sep 15;15(9):1935. doi: 10.3390/v15091935. Viruses. 2023. PMID: 37766341 Free PMC article. Review.
References
-
- Polizzotto M.N., Uldrick T.S., Wyvill K.M., Aleman K., Marshall V., Wang V., Whitby D., Pittaluga S., Jaffe E.S., Millo C., et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS) Clin. Infect. Dis. 2015;62:730–738. doi: 10.1093/cid/civ996. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

