Emerging Trends in Biodegradable Microcarriers for Therapeutic Applications
- PMID: 36987266
- PMCID: PMC10057597
- DOI: 10.3390/polym15061487
Emerging Trends in Biodegradable Microcarriers for Therapeutic Applications
Abstract
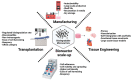
Microcarriers (MCs) are adaptable therapeutic instruments that may be adjusted to specific therapeutic uses, making them an appealing alternative for regenerative medicine and drug delivery. MCs can be employed to expand therapeutic cells. MCs can be used as scaffolds for tissue engineering, as well as providing a 3D milieu that replicates the original extracellular matrix, facilitating cell proliferation and differentiation. Drugs, peptides, and other therapeutic compounds can be carried by MCs. The surface of the MCs can be altered, to improve medication loading and release, and to target specific tissues or cells. Allogeneic cell therapies in clinical trials require enormous volumes of stem cells, to assure adequate coverage for several recruitment locations, eliminate batch to batch variability, and reduce production costs. Commercially available microcarriers necessitate additional harvesting steps to extract cells and dissociation reagents, which reduces cell yield and quality. To circumvent such production challenges, biodegradable microcarriers have been developed. In this review, we have compiled key information relating to biodegradable MC platforms, for generating clinical-grade cells, that permit cell delivery at the target site without compromising quality or cell yields. Biodegradable MCs could also be employed as injectable scaffolds for defect filling, supplying biochemical signals for tissue repair and regeneration. Bioinks, coupled with biodegradable microcarriers with controlled rheological properties, might improve bioactive profiles, while also providing mechanical stability to 3D bioprinted tissue structures. Biodegradable materials used for microcarriers have the ability to solve in vitro disease modeling, and are advantageous to the biopharmaceutical drug industries, because they widen the spectrum of controllable biodegradation and may be employed in a variety of applications.
Keywords: biodegradable; cell manufacturing; cell therapy; mesenchymal stem cells; microcarriers; regenerative medicine; stem cells.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors.Cytotherapy. 2017 Mar;19(3):419-432. doi: 10.1016/j.jcyt.2016.11.009. Epub 2016 Dec 22. Cytotherapy. 2017. PMID: 28017598
-
Sub-confluent culture of human mesenchymal stromal cells on biodegradable polycaprolactone microcarriers enhances bone healing of rat calvarial defect.Cytotherapy. 2019 Jun;21(6):631-642. doi: 10.1016/j.jcyt.2019.03.004. Epub 2019 Apr 8. Cytotherapy. 2019. PMID: 30975604
-
Enhanced in vitro osteogenic differentiation of human fetal MSCs attached to 3D microcarriers versus harvested from 2D monolayers.BMC Biotechnol. 2015 Oct 31;15:102. doi: 10.1186/s12896-015-0219-8. BMC Biotechnol. 2015. PMID: 26520400 Free PMC article.
-
Functional cells cultured on microcarriers for use in regenerative medicine research.Cell Transplant. 2011;20(1):49-62. doi: 10.3727/096368910X532792. Epub 2010 Sep 30. Cell Transplant. 2011. PMID: 20887678 Review.
-
Microcarriers in application for cartilage tissue engineering: Recent progress and challenges.Bioact Mater. 2022 Jan 25;17:81-108. doi: 10.1016/j.bioactmat.2022.01.033. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386447 Free PMC article. Review.
Cited by
-
A review on selenium and gold nanoparticles combined photodynamic and photothermal prostate cancer tumors ablation.Discov Nano. 2023 Dec 7;18(1):150. doi: 10.1186/s11671-023-03936-z. Discov Nano. 2023. PMID: 38062330 Free PMC article. Review.
References
-
- Farid S.S., Jenkins M.J. Chapter 44—Bioprocesses for Cell Therapies. In: Jagschies G., Lindskog E., Łącki K., Galliher P., editors. Biopharmaceutical Processing. Elsevier; Amsterdam, The Netherlands: 2018. pp. 899–930. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

