5,8-Dimethyl-9 H-carbazole Derivatives Blocking hTopo I Activity and Actin Dynamics
- PMID: 36986453
- PMCID: PMC10051477
- DOI: 10.3390/ph16030353
5,8-Dimethyl-9 H-carbazole Derivatives Blocking hTopo I Activity and Actin Dynamics
Abstract
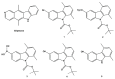
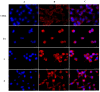
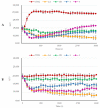
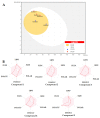
Over the years, carbazoles have been largely studied for their numerous biological properties, including antibacterial, antimalarial, antioxidant, antidiabetic, neuroprotective, anticancer, and many more. Some of them have gained great interest for their anticancer activity in breast cancer due to their capability in inhibiting essential DNA-dependent enzymes, namely topoisomerases I and II. With this in mind, we studied the anticancer activity of a series of carbazole derivatives against two breast cancer cell lines, namely the triple negative MDA-MB-231 and MCF-7 cells. Compounds 3 and 4 were found to be the most active towards the MDA-MB-231 cell line without interfering with the normal counterpart. Using docking simulations, we assessed the ability of these carbazole derivatives to bind human topoisomerases I and II and actin. In vitro specific assays confirmed that the lead compounds selectively inhibited the human topoisomerase I and interfered with the normal organization of the actin system, triggering apoptosis as a final effect. Thus, compounds 3 and 4 are strong candidates for further drug development in multi-targeted therapy for the treatment of triple negative breast cancer, for which safe therapeutic regimens are not yet available.
Keywords: actin dynamics; anticancer; docking simulation; human topoisomerases I/II.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Inhibition of Human Topoisomerase II by N,N,N-Trimethylethanammonium Iodide Alkylcarbazole Derivatives.ChemMedChem. 2018 Dec 20;13(24):2635-2643. doi: 10.1002/cmdc.201800546. Epub 2018 Nov 30. ChemMedChem. 2018. PMID: 30347518
-
Novel Thiazolidine-2,4-dione-trimethoxybenzene-thiazole Hybrids as Human Topoisomerases Inhibitors.Pharmaceuticals (Basel). 2023 Jun 29;16(7):946. doi: 10.3390/ph16070946. Pharmaceuticals (Basel). 2023. PMID: 37513858 Free PMC article.
-
Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors.Eur J Pharm Sci. 2017 Jan 1;96:263-272. doi: 10.1016/j.ejps.2016.09.039. Epub 2016 Oct 1. Eur J Pharm Sci. 2017. PMID: 27702608
-
In-vitro Evaluation of Isatin Derivatives as Potent Anti-Breast Cancer Agents against MCF-7, MDA MB 231, MDA-MB 435 and MDA-MB 468 Breast Cancers Cell Lines: A Review.Anticancer Agents Med Chem. 2022;22(10):1883-1896. doi: 10.2174/1871520621666210903130152. Anticancer Agents Med Chem. 2022. PMID: 34477529 Review.
-
Natural Products as Anti-Cancerous Therapeutic Molecules Targeted towards Topoisomerases.Curr Protein Pept Sci. 2020;21(11):1103-1142. doi: 10.2174/1389203721666200918152511. Curr Protein Pept Sci. 2020. PMID: 32951576 Review.
Cited by
-
Understanding Cancer's Defense against Topoisomerase-Active Drugs: A Comprehensive Review.Cancers (Basel). 2024 Feb 6;16(4):680. doi: 10.3390/cancers16040680. Cancers (Basel). 2024. PMID: 38398072 Free PMC article. Review.
References
-
- Caruso A., Chimento A., El-Kashef H., Lancelot J.-C., Panno A., Pezzi V., Saturnino C., Sinicropi M.S., Sirianni R., Rault S. Antiproliferative activity of some 1,4-dimethylcarbazoles on cells that express estrogen receptors: Part I. J. Enzym. Inhib. Med. Chem. 2012;27:609–613. doi: 10.3109/14756366.2011.603132. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

