Characterization of AtBAG2 as a Novel Molecular Chaperone
- PMID: 36983842
- PMCID: PMC10052705
- DOI: 10.3390/life13030687
Characterization of AtBAG2 as a Novel Molecular Chaperone
Abstract
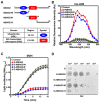
Bcl-2-associated anthanogene (BAG) family proteins regulate plant defense against biotic and abiotic stresses; however, the function and precise mechanism of action of each individual BAG protein are not yet clear. In this study, we investigated the biochemical and molecular functions of the Arabidopsis thaliana BAG2 (AtBAG2) protein, and elucidated its physiological role under stress conditions using mutant plants and transgenic yeast strains. The T-DNA insertion atbag2 mutant plants were highly susceptible to heat shock, whereas transgenic yeast strains ectopically expressing AtBAG2 exhibited outstanding thermotolerance. Moreover, a biochemical analysis of GST-fused recombinant proteins produced in bacteria revealed that AtBAG2 exhibits molecular chaperone activity, which could be attributed to its BAG domain. The relevance of the molecular chaperone function of AtBAG2 to the cellular heat stress response was confirmed using yeast transformants, and the experimental results showed that overexpression of the AtBAG2 sequence encoding only the BAG domain was sufficient to impart thermotolerance. Overall, these results suggest that the BAG domain-dependent molecular chaperone activity of AtBAG2 is indispensable for the heat stress response of Arabidopsis. This is the first report demonstrating the role of AtBAG2 as a sole molecular chaperone in Arabidopsis.
Keywords: BAG (Bcl-2-associated anthanogene) family proteins; abiotic stress; molecular chaperone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Heat-induced chaperone activity of serine/threonine protein phosphatase 5 enhances thermotolerance in Arabidopsis thaliana.New Phytol. 2011 Aug;191(3):692-705. doi: 10.1111/j.1469-8137.2011.03734.x. Epub 2011 May 12. New Phytol. 2011. PMID: 21564098
-
The BAG2 and BAG6 Genes Are Involved in Multiple Abiotic Stress Tolerances in Arabidopsis Thaliana.Int J Mol Sci. 2021 May 29;22(11):5856. doi: 10.3390/ijms22115856. Int J Mol Sci. 2021. PMID: 34072612 Free PMC article.
-
Structural insight into plant programmed cell death mediated by BAG proteins in Arabidopsis thaliana.Acta Crystallogr D Biol Crystallogr. 2013 Jun;69(Pt 6):934-45. doi: 10.1107/S0907444913003624. Epub 2013 May 2. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 23695238
-
Functional insights of plant bcl-2-associated ahanogene (BAG) proteins: Multi-taskers in diverse cellular signal transduction pathways.Front Plant Sci. 2023 Mar 28;14:1136873. doi: 10.3389/fpls.2023.1136873. eCollection 2023. Front Plant Sci. 2023. PMID: 37056491 Free PMC article. Review.
-
Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses.Sci Total Environ. 2022 Jun 15;825:154054. doi: 10.1016/j.scitotenv.2022.154054. Epub 2022 Feb 21. Sci Total Environ. 2022. PMID: 35202686 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

