Aminooxy Click Modification of a Periodate-Oxidized Immunoglobulin G: A General Approach to Antibody-Drug Conjugates with Dye-Mediated Expeditious Stoichiometry Control
- PMID: 36982208
- PMCID: PMC10049567
- DOI: 10.3390/ijms24065134
Aminooxy Click Modification of a Periodate-Oxidized Immunoglobulin G: A General Approach to Antibody-Drug Conjugates with Dye-Mediated Expeditious Stoichiometry Control
Abstract
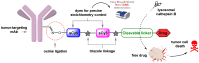
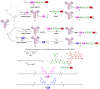
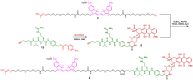
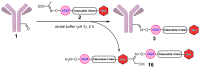
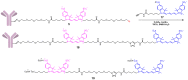
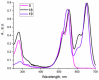
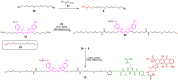
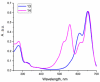
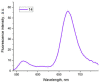
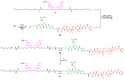
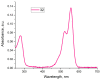
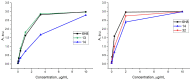
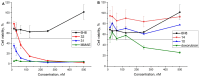
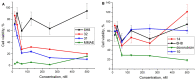
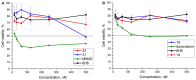
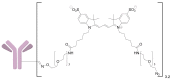
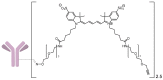
A universal approach to the construction of antibody-drug conjugates (ADCs) has been developed. It relies on periodate oxidation of naturally present glycans of immunoglobulin G, followed by oxime ligation and, optionally, copper(I)-catalyzed alkyne-azide cycloaddition for conjugation with a toxic payload. The introduction of highly absorbing cyanine dyes into the linker allows for facile determination of the drug-antibody ratio. We applied this methodology to the synthesis of cytotoxic conjugates of an antibody against the tumor-associated antigen PRAME with doxorubicin and monomethyl auristatin E (MMAE). The resultant conjugates retained their affinity to a large extent, yet their cytotoxicity in vitro varied dramatically: while the doxorubicin-based conjugate did not produce any effect on cells, the MMAE-based one demonstrated specific activity against PRAME-expressing cancer cell lines. Importantly, the latter conjugate constitutes the first reported example of a PRAME-targeting ADC.
Keywords: ADC; CuAAC; MMAE; PRAME; antibody; cleavable linkers; cyanine dyes; doxorubicin; oxime ligation; periodate oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

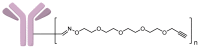

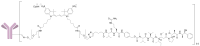

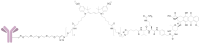
Similar articles
-
A Novel Anti-CD22 Anthracycline-Based Antibody-Drug Conjugate (ADC) That Overcomes Resistance to Auristatin-Based ADCs.Clin Cancer Res. 2015 Jul 15;21(14):3298-306. doi: 10.1158/1078-0432.CCR-14-2035. Epub 2015 Apr 3. Clin Cancer Res. 2015. PMID: 25840969
-
Development and Properties of Valine-Alanine based Antibody-Drug Conjugates with Monomethyl Auristatin E as the Potent Payload.Int J Mol Sci. 2017 Aug 25;18(9):1860. doi: 10.3390/ijms18091860. Int J Mol Sci. 2017. PMID: 28841157 Free PMC article.
-
Sensitive Immunofluorescent Detection of the PRAME Antigen Using a Practical Antibody Conjugation Approach.Int J Mol Sci. 2021 Nov 27;22(23):12845. doi: 10.3390/ijms222312845. Int J Mol Sci. 2021. PMID: 34884647 Free PMC article.
-
Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives.Chem Pharm Bull (Tokyo). 2020;68(3):201-211. doi: 10.1248/cpb.c19-00853. Chem Pharm Bull (Tokyo). 2020. PMID: 32115527 Review.
-
Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985).Eur J Med Chem. 2019 Dec 1;183:111682. doi: 10.1016/j.ejmech.2019.111682. Epub 2019 Sep 6. Eur J Med Chem. 2019. PMID: 31563805 Review.
Cited by
-
Introduction of Carbonyl Groups into Antibodies.Molecules. 2023 Dec 1;28(23):7890. doi: 10.3390/molecules28237890. Molecules. 2023. PMID: 38067618 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

