The Impact of TRAIL on the Immunological Milieu during the Early Stage of Abdominal Sepsis
- PMID: 36980658
- PMCID: PMC10046128
- DOI: 10.3390/cancers15061773
The Impact of TRAIL on the Immunological Milieu during the Early Stage of Abdominal Sepsis
Abstract
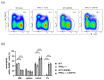
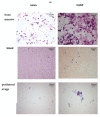
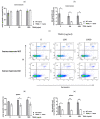
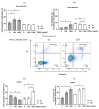
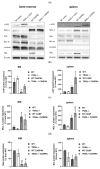
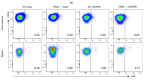
Despite intensive scientific efforts, the therapy of peritonitis is presently limited to symptomatic measures, including infectious source control and broad-spectrum antibiotics. Promising therapeutic approaches to reduce morbidity and mortality are still missing. Within the early phase of abdominal sepsis, apoptosis of neutrophil granulocytes is inhibited, which is linked to tissue damage and septic shock. TNF-related apoptosis-inducing ligand (TRAIL) is a promising agent to stimulate neutrophil apoptosis. However, the underlying mechanisms have not been elucidated so far. The objective of the present study was to characterize the molecular mechanisms of TRAIL-stimulated apoptosis in early abdominal sepsis. Therefore, the murine sepsis model Colon ascendens stent peritonitis (CASP) was applied in wild type (WT) and TRAIL knock-out (TRAIL-/-) C57/BL6j mice. Neutrophil granulocytes were isolated from spleen, blood, bone marrow, and peritoneal lavage using magnetic-activated cell sorting. Neutrophil maturation was analyzed by light microscopy, and apoptotic neutrophils were quantified by fluorescence-activated cell sorting (FACS). Western blot and FACS were used to investigate expression changes in apoptotic proteins and TRAIL receptors. The impact of TRAIL-induced apoptosis was studied in vitro. In septic mice (CASP 6 h), the number of neutrophils in the BM was reduced but increased in the blood and peritoneal lavage. This was paralleled by an increased maturation of neutrophils from rod-shaped to segmented neutrophils (right shift). In vitro, extrinsic TRAIL stimulation did not alter the apoptosis level of naïve neutrophils but stimulated apoptosis in neutrophils derived from septic WT and TRAIL-/- mice. Neutrophils of the bone marrow and spleen showed enhanced protein expression of anti-apoptotic Flip, c-IAP1, and McL-1 and reduced expression levels of pro-apoptotic Bax in neutrophils, which might correlate with apoptosis inhibition in these cells. CASP increased the expression of intrinsic TRAIL in neutrophils derived from the bone marrow and spleen. This might be explained by an increased expression of the TRAIL receptors DR5, DcR1, and DcR2 on neutrophils in sepsis. No differences were observed between septic or naïve WT and TRAIL-/- mice. In conclusion, the present study shows that neutrophil granulocytes are sensitive to TRAIL-stimulated apoptosis in the early stage of abdominal sepsis, emphasizing the promising role of TRAIL as a therapeutic agent.
Keywords: CASP; TRAIL; apoptosis; neutrophils; peritonitis; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
TRAIL induces neutrophil apoptosis and dampens sepsis-induced organ injury in murine colon ascendens stent peritonitis.PLoS One. 2014 Jun 2;9(6):e97451. doi: 10.1371/journal.pone.0097451. eCollection 2014. PLoS One. 2014. PMID: 24887152 Free PMC article.
-
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) improves the innate immune response and enhances survival in murine polymicrobial sepsis.Crit Care Med. 2010 Nov;38(11):2169-74. doi: 10.1097/CCM.0b013e3181eedaa8. Crit Care Med. 2010. PMID: 20657274
-
TNF-related apoptosis-inducing ligand deficiency enhances survival in murine colon ascendens stent peritonitis.J Inflamm Res. 2016 Jun 16;9:103-13. doi: 10.2147/JIR.S99887. eCollection 2016. J Inflamm Res. 2016. PMID: 27366100 Free PMC article.
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
-
E3 ubiquitin ligases and deubiquitinases as modulators of TRAIL-mediated extrinsic apoptotic signaling pathway.BMB Rep. 2019 Feb;52(2):119-126. doi: 10.5483/BMBRep.2019.52.2.011. BMB Rep. 2019. PMID: 30638181 Free PMC article. Review.
Cited by
-
Gut microbiota, circulating inflammatory proteins and sepsis: a bi-directional Mendelian randomization study.Front Cell Infect Microbiol. 2024 Aug 8;14:1398756. doi: 10.3389/fcimb.2024.1398756. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39176264 Free PMC article.
References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Novotny A.R., Reim D., Assfalg V., Altmayr F., Friess H.M., Emmanuel K., Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616–621. doi: 10.1016/j.imbio.2011.10.019. - DOI - PubMed
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

