Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics
- PMID: 36980629
- PMCID: PMC10046032
- DOI: 10.3390/cancers15061743
Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics
Abstract
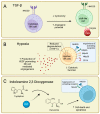
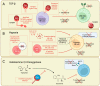
Natural killer (NK) cells are cytotoxic group 1 innate lymphoid cells (ILC), known for their role as killers of stressed, cancerous, and virally infected cells. Beyond this cytotoxic function, NK cell subsets can influence broader immune responses through cytokine production and have been linked to central roles in non-immune processes, such as the regulation of vascular remodeling in pregnancy and cancer. Attempts to exploit the anti-tumor functions of NK cells have driven the development of various NK cell-based therapies, which have shown promise in both pre-clinical disease models and early clinical trials. However, certain elements of the tumor microenvironment, such as elevated transforming growth factor (TGF)-β, hypoxia, and indoalemine-2,3-dioxygenase (IDO), are known to suppress NK cell function, potentially limiting the longevity and activity of these approaches. Recent studies have also identified these factors as contributors to NK cell plasticity, defined by the conversion of classical cytotoxic NK cells into poorly cytotoxic, tissue-resident, or ILC1-like phenotypes. This review summarizes the current approaches for NK cell-based cancer therapies and examines the challenges presented by tumor-linked NK cell suppression and plasticity. Ongoing efforts to overcome these challenges are discussed, along with the potential utility of NK cell therapies to applications outside cancer.
Keywords: HIF1α; NK cell dysfunction; NK cell plasticity; NK cell therapeutics; TGFβ; indoalemine-2,3-dioxygenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Hitting More Birds with a Stone: Impact of TGF-β on ILC Activity in Cancer.J Clin Med. 2020 Jan 5;9(1):143. doi: 10.3390/jcm9010143. J Clin Med. 2020. PMID: 31948072 Free PMC article. Review.
-
Plasticity of NK cells in Cancer.Front Immunol. 2022 May 10;13:888313. doi: 10.3389/fimmu.2022.888313. eCollection 2022. Front Immunol. 2022. PMID: 35619715 Free PMC article. Review.
-
CIS and TGF-β regulatory pathways influence immunity to bacterial infection.Immunology. 2022 Sep;167(1):54-63. doi: 10.1111/imm.13516. Epub 2022 Jun 22. Immunology. 2022. PMID: 35611558
-
The Production of Pro-angiogenic VEGF-A Isoforms by Hypoxic Human NK Cells Is Independent of Their TGF-β-Mediated Conversion to an ILC1-Like Phenotype.Front Immunol. 2020 Aug 25;11:1903. doi: 10.3389/fimmu.2020.01903. eCollection 2020. Front Immunol. 2020. PMID: 32983113 Free PMC article.
-
NK cell-based therapeutics for lung cancer.Expert Opin Biol Ther. 2020 Jan;20(1):23-33. doi: 10.1080/14712598.2020.1688298. Epub 2019 Nov 12. Expert Opin Biol Ther. 2020. PMID: 31714156 Review.
Cited by
-
Forks in the road for CAR T and CAR NK cell cancer therapies.Nat Immunol. 2023 Dec;24(12):1994-2007. doi: 10.1038/s41590-023-01659-y. Epub 2023 Nov 27. Nat Immunol. 2023. PMID: 38012406 Review.
-
The ovarian cancer-associated microbiome contributes to the tumor's inflammatory microenvironment.Front Cell Infect Microbiol. 2024 Oct 21;14:1440742. doi: 10.3389/fcimb.2024.1440742. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39497925 Free PMC article. Review.
-
Cancer Immunotherapy: Where Next?Cancers (Basel). 2023 Apr 18;15(8):2358. doi: 10.3390/cancers15082358. Cancers (Basel). 2023. PMID: 37190286 Free PMC article. Review.
-
Immunometabolism: signaling pathways, homeostasis, and therapeutic targets.MedComm (2020). 2024 Nov 3;5(11):e789. doi: 10.1002/mco2.789. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492834 Free PMC article. Review.
-
Natural killer cell therapies.Nature. 2024 Feb;626(8000):727-736. doi: 10.1038/s41586-023-06945-1. Epub 2024 Feb 21. Nature. 2024. PMID: 38383621 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

