The Similar and Distinct Roles of Satellite Glial Cells and Spinal Astrocytes in Neuropathic Pain
- PMID: 36980304
- PMCID: PMC10047571
- DOI: 10.3390/cells12060965
The Similar and Distinct Roles of Satellite Glial Cells and Spinal Astrocytes in Neuropathic Pain
Abstract
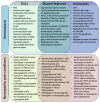
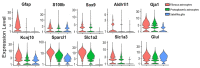
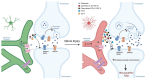
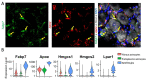
Preclinical studies have identified glial cells as pivotal players in the genesis and maintenance of neuropathic pain after nerve injury associated with diabetes, chemotherapy, major surgeries, and virus infections. Satellite glial cells (SGCs) in the dorsal root and trigeminal ganglia of the peripheral nervous system (PNS) and astrocytes in the central nervous system (CNS) express similar molecular markers and are protective under physiological conditions. They also serve similar functions in the genesis and maintenance of neuropathic pain, downregulating some of their homeostatic functions and driving pro-inflammatory neuro-glial interactions in the PNS and CNS, i.e., "gliopathy". However, the role of SGCs in neuropathic pain is not simply as "peripheral astrocytes". We delineate how these peripheral and central glia participate in neuropathic pain by producing different mediators, engaging different parts of neurons, and becoming active at different stages following nerve injury. Finally, we highlight the recent findings that SGCs are enriched with proteins related to fatty acid metabolism and signaling such as Apo-E, FABP7, and LPAR1. Targeting SGCs and astrocytes may lead to novel therapeutics for the treatment of neuropathic pain.
Keywords: dorsal root ganglia; fatty acids; gliopathy; nerve injury; spinal cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Satellite glial cells in sensory ganglia express functional transient receptor potential ankyrin 1 that is sensitized in neuropathic and inflammatory pain.Mol Pain. 2020 Jan-Dec;16:1744806920925425. doi: 10.1177/1744806920925425. Mol Pain. 2020. PMID: 32484015 Free PMC article.
-
Satellite glial cell-secreted exosomes after in-vitro oxaliplatin treatment presents a pro-nociceptive effect for dorsal root ganglion neurons and induce mechanical hypersensitivity in naïve mice.Mol Cell Neurosci. 2023 Sep;126:103881. doi: 10.1016/j.mcn.2023.103881. Epub 2023 Jul 18. Mol Cell Neurosci. 2023. PMID: 37467904
-
TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord.Spine (Phila Pa 1976). 2004 May 15;29(10):1082-8. doi: 10.1097/00007632-200405150-00006. Spine (Phila Pa 1976). 2004. PMID: 15131433
-
The role of satellite glial cells in orofacial pain.J Neurosci Res. 2019 Apr;97(4):393-401. doi: 10.1002/jnr.24341. Epub 2018 Nov 19. J Neurosci Res. 2019. PMID: 30450738 Review.
-
New Insights on the Role of Satellite Glial Cells.Stem Cell Rev Rep. 2023 Feb;19(2):358-367. doi: 10.1007/s12015-022-10460-7. Epub 2022 Oct 15. Stem Cell Rev Rep. 2023. PMID: 36242721 Review.
Cited by
-
Communicating pain: emerging axonal signaling in peripheral neuropathic pain.Front Neuroanat. 2024 Jul 9;18:1398400. doi: 10.3389/fnana.2024.1398400. eCollection 2024. Front Neuroanat. 2024. PMID: 39045347 Free PMC article. Review.
-
Pain research in a petri dish? Advantages and limitations of neuro-glial primary cell cultures from structures of the nociceptive system.Brain Behav Immun Health. 2024 Sep 6;41:100854. doi: 10.1016/j.bbih.2024.100854. eCollection 2024 Nov. Brain Behav Immun Health. 2024. PMID: 39308957 Free PMC article.
-
Characterisation of GFAP-Expressing Glial Cells in the Dorsal Root Ganglion after Spared Nerve Injury.Int J Mol Sci. 2023 Oct 25;24(21):15559. doi: 10.3390/ijms242115559. Int J Mol Sci. 2023. PMID: 37958541 Free PMC article.
-
Transcriptional profiles of non-neuronal and immune cells in mouse trigeminal ganglia.Front Pain Res (Lausanne). 2023 Oct 31;4:1274811. doi: 10.3389/fpain.2023.1274811. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 38028432 Free PMC article.
-
Sigma-1 Receptor Inhibition Reduces Mechanical Allodynia and Modulate Neuroinflammation in Chronic Neuropathic Pain.Mol Neurobiol. 2024 May;61(5):2672-2685. doi: 10.1007/s12035-023-03717-w. Epub 2023 Nov 3. Mol Neurobiol. 2024. PMID: 37922065 Free PMC article.
References
-
- Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

