Action of 2,6-Dichloro-1,4-benzoquinone on the O2-Evolving Activity of Photosystem II in Chlamydomonas reinhardtii Cells with and without Cell Wall: Inhibitory Effect of Its Oxidized Form
- PMID: 36980248
- PMCID: PMC10046965
- DOI: 10.3390/cells12060907
Action of 2,6-Dichloro-1,4-benzoquinone on the O2-Evolving Activity of Photosystem II in Chlamydomonas reinhardtii Cells with and without Cell Wall: Inhibitory Effect of Its Oxidized Form
Abstract
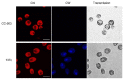
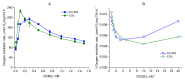
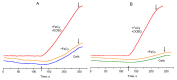
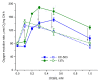
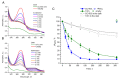
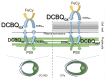
Chlamydomonas reinhardtii is a widely used object in studies on green algae concerning both photosynthesis aspects and possible biotechnological approaches. The measurement of the maximum O2 evolution by photosystem II (PSII) in living algal cells in the presence of artificial acceptors is one of the commonly used methods for determining the photosynthetic apparatus state or its change as compared to a control, parent strain, etc., because PSII is the most sensitive component of the thylakoid membrane. The present study shows the need to use low concentrations of 2,6-dichloro-1,4-benzoquinone (DCBQ) paired with potassium ferricyanide (FeCy) for achieving the maximum O2 evolution rate, while a DCBQ concentration above certain threshold results in strong suppression of O2 evolution. The required DCBQ concentration depends on the presence of the cell wall and should be exactly ~0.1 mM or in the range of 0.2-0.4 mM for cells with and without a cell wall, respectively. The inhibition effect is caused, probably, by a higher content of DCBQ in the oxidized form inside cells; this depends on the presence of the cell wall, which influences the efficiency of DCBQ diffusion into and out of the cell, where it is maintained by FeCy in the oxidized state. The possible mechanism of DCBQ inhibition action is discussed.
Keywords: 2,6-dichloro-1,4-benzoquinone (DCBQ); Chlamydomonas; O2 evolution rate; cell wall; photosystem II.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CAH3 from Chlamydomonas reinhardtii: Unique Carbonic Anhydrase of the Thylakoid Lumen.Cells. 2024 Jan 5;13(2):109. doi: 10.3390/cells13020109. Cells. 2024. PMID: 38247801 Free PMC article. Review.
-
Acetate in mixotrophic growth medium affects photosystem II in Chlamydomonas reinhardtii and protects against photoinhibition.Biochim Biophys Acta. 2013 Oct;1827(10):1183-90. doi: 10.1016/j.bbabio.2013.06.004. Epub 2013 Jun 17. Biochim Biophys Acta. 2013. PMID: 23791666
-
Investigation of the electron transfer site of p-benzoquinone in isolated photosystem II particles and thylakoid membranes using alpha- and beta-cyclodextrins.J Photochem Photobiol B. 2006 Dec 1;85(3):177-83. doi: 10.1016/j.jphotobiol.2006.07.003. Epub 2006 Aug 24. J Photochem Photobiol B. 2006. PMID: 16934484
-
The mechanism of anthracene interaction with photosynthetic apparatus: a study using intact cells, thylakoid membranes and PS II complexes isolated from Chlamydomonas reinhardtii.Aquat Toxicol. 2011 Aug;104(3-4):205-10. doi: 10.1016/j.aquatox.2011.04.017. Epub 2011 May 6. Aquat Toxicol. 2011. PMID: 21632024
-
Thylakoid membrane dynamics and state transitions in Chlamydomonas reinhardtii under elevated temperature.Photosynth Res. 2019 Mar;139(1-3):215-226. doi: 10.1007/s11120-018-0562-4. Epub 2018 Jul 20. Photosynth Res. 2019. PMID: 30030686
Cited by
-
CAH3 from Chlamydomonas reinhardtii: Unique Carbonic Anhydrase of the Thylakoid Lumen.Cells. 2024 Jan 5;13(2):109. doi: 10.3390/cells13020109. Cells. 2024. PMID: 38247801 Free PMC article. Review.
References
-
- Suzuki T., Minagawa J., Tomo T., Sonoike K., Ohta H., Enami I. Binding and Functional Properties of the Extrinsic Proteins in Oxygen-Evolving Photosystem II Particle from a Green Alga, Chlamydomonas Reinhardtii Having His-Tagged CP47. Plant Cell Physiol. 2003;44:76–84. doi: 10.1093/pcp/pcg010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

