The Inhibition Effects of Sodium Nitroprusside on the Survival of Differentiated Neural Stem Cells through the p38 Pathway
- PMID: 36979248
- PMCID: PMC10046126
- DOI: 10.3390/brainsci13030438
The Inhibition Effects of Sodium Nitroprusside on the Survival of Differentiated Neural Stem Cells through the p38 Pathway
Abstract
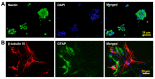
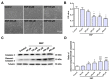
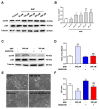
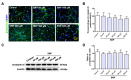
Nitric oxide (NO) is a crucial factor in regulating neuronal development. However, certain effects of NO are complex under different physiological conditions. In this study, we used differentiated neural stem cells (NSCs), which contained neural progenitor cells, neurons, astrocytes, and oligodendrocytes, to observe the physiological effects of sodium nitroprusside (SNP) on the early developmental stage of the nervous system. After SNP treatment for 24 h, the results showed that SNP at 100 μM, 200 μM, 300 μM, and 400 μM concentrations resulted in reduced cell viability and increased cleaved caspase 3 levels, while no significant changes were found at 50 μM. There were no effects on neuronal differentiation in the SNP-treated groups. The phosphorylation of p38 was also significantly upregulated with SNP concentrations of 100 μM, 200 μM, 300 μM, and 400 μM, with no changes for 50 μM concentration in comparison with the control. We also observed that the levels of phosphorylation increased with the increasing concentration of SNP. To further explore the possible role of p38 in SNP-regulated survival of differentiated NSCs, SB202190, the antagonist of p38 mitogen-activated protein kinase, at a concentration of 10 mM, was pretreated for 30 min, and the ratio of phosphorylated p38 was found to be decreased after treatment with SNP. Survival and cell viability increased in the SB202190 and SNP co-treated group. Taken together, our results suggested that p38 is involved in the cell survival of NSCs, regulated by NO.
Keywords: differentiation; neural stem cells; sodium nitroprusside; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sodium nitroprusside activates p38 mitogen activated protein kinase through a cGMP/PKG independent mechanism.Life Sci. 2007 Aug 2;81(8):640-6. doi: 10.1016/j.lfs.2007.06.022. Epub 2007 Jul 5. Life Sci. 2007. PMID: 17707440
-
The action of nitric oxide to enhance cell survival in chick cardiomyocytes is mediated through a cGMP and ERK1/2 pathway while p38 mitogen-activated protein kinase-dependent pathways do not alter cell death.Exp Physiol. 2008 Jul;93(7):834-42. doi: 10.1113/expphysiol.2008.042176. Epub 2008 Mar 14. Exp Physiol. 2008. PMID: 18344257
-
Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger.Glia. 2007 Oct;55(13):1325-33. doi: 10.1002/glia.20541. Glia. 2007. PMID: 17626263
-
ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status.J Biol Chem. 2002 Jan 11;277(2):1332-9. doi: 10.1074/jbc.M107231200. Epub 2001 Oct 31. J Biol Chem. 2002. PMID: 11689560
-
Sodium nitroprusside-induced mitochondrial apoptotic events in insulin-secreting RINm5F cells are associated with MAP kinases activation.Exp Cell Res. 2001 Oct 1;269(2):222-9. doi: 10.1006/excr.2001.5315. Exp Cell Res. 2001. PMID: 11570814
References
-
- Carreira B.P., Morte M.I., Inacio A., Costa G., Rosmaninho-Salgado J., Agasse F., Carmo A., Couceiro P., Brundin P., Ambrosio A.F., et al. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells. 2010;28:1219–1230. doi: 10.1002/stem.444. - DOI - PubMed
-
- Moreno-Lopez B., Romero-Grimaldi C., Noval J.A., Murillo-Carretero M., Matarredona E.R., Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J. Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

