trans-Cyclooctene- and Bicyclononyne-Linked Nucleotides for Click Modification of DNA with Fluorogenic Tetrazines and Live Cell Metabolic Labeling and Imaging
- PMID: 36972479
- PMCID: PMC10119924
- DOI: 10.1021/acs.bioconjchem.3c00064
trans-Cyclooctene- and Bicyclononyne-Linked Nucleotides for Click Modification of DNA with Fluorogenic Tetrazines and Live Cell Metabolic Labeling and Imaging
Abstract
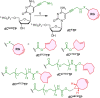
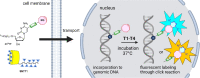
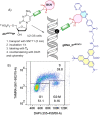
A series of 2'-deoxyribonucleoside triphosphates (dNTPs) bearing 2- or 4-linked trans-cyclooctene (TCO) or bicyclononyne (BCN) tethered through a shorter propargylcarbamate or longer triethyleneglycol-based spacer were designed and synthesized. They were found to be good substrates for KOD XL DNA polymerase for primer extension enzymatic synthesis of modified oligonucleotides. We systematically tested and compared the reactivity of TCO- and BCN-modified nucleotides and DNA with several fluorophore-containing tetrazines in inverse electron-demand Diels-Alder (IEDDA) click reactions to show that the longer linker is crucial for efficient labeling. The modified dNTPs were transported into live cells using the synthetic transporter SNTT1, incubated for 1 h, and then treated with tetrazine conjugates. The PEG3-linked 4TCO and BCN nucleotides showed efficient incorporation into genomic DNA and good reactivity in the IEDDA click reaction with tetrazines to allow staining of DNA and imaging of DNA synthesis in live cells within time periods as short as 15 min. The BCN-linked nucleotide in combination with TAMRA-linked (TAMRA = carboxytetramethylrhodamine) tetrazine was also efficiently used for staining of DNA for flow cytometry. This methodology is a new approach for in cellulo metabolic labeling and imaging of DNA synthesis which is shorter, operationally simple, and overcomes several problems of previously used methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Clip to Click: Controlling Inverse Electron-Demand Diels-Alder Reactions with Macrocyclic Tetrazines.Org Lett. 2022 May 6;24(17):3223-3226. doi: 10.1021/acs.orglett.2c01010. Epub 2022 Apr 21. Org Lett. 2022. PMID: 35446571
-
Development of a novel antibody-tetrazine conjugate for bioorthogonal pretargeting.Org Biomol Chem. 2016 Aug 21;14(31):7544-51. doi: 10.1039/c6ob01411a. Epub 2016 Jul 19. Org Biomol Chem. 2016. PMID: 27431745
-
Secondary Orbital Interactions Enhance the Reactivity of Alkynes in Diels-Alder Cycloadditions.J Am Chem Soc. 2019 Feb 13;141(6):2224-2227. doi: 10.1021/jacs.8b13088. Epub 2019 Jan 31. J Am Chem Soc. 2019. PMID: 30693769 Free PMC article.
-
Inverse electron demand Diels-Alder reactions in chemical biology.Chem Soc Rev. 2017 Aug 14;46(16):4895-4950. doi: 10.1039/c7cs00184c. Chem Soc Rev. 2017. PMID: 28660957 Review.
-
IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications.Molecules. 2021 Jul 30;26(15):4640. doi: 10.3390/molecules26154640. Molecules. 2021. PMID: 34361793 Free PMC article. Review.
Cited by
-
Bioorthogonal Chemistry in Cellular Organelles.Top Curr Chem (Cham). 2023 Dec 16;382(1):2. doi: 10.1007/s41061-023-00446-5. Top Curr Chem (Cham). 2023. PMID: 38103067 Free PMC article. Review.
-
Metabolic labelling of DNA in cells by means of the "photoclick" reaction triggered by visible light.RSC Chem Biol. 2023 Aug 30;4(12):1037-1042. doi: 10.1039/d3cb00150d. eCollection 2023 Nov 29. RSC Chem Biol. 2023. PMID: 38033731 Free PMC article.
References
-
- Güixens-Gallardo P.; Zawada Z.; Matyašovský J.; Dziuba D.; Pohl R.; Kraus T.; Hocek M. Brightly Fluorescent 2′-Deoxyribonucleoside Triphosphates Bearing Methylated Bodipy Fluorophore for in Cellulo Incorporation to DNA, Imaging, and Flow Cytometry. Bioconjugate Chem. 2018, 29, 3906–3912. 10.1021/acs.bioconjchem.8b00721. - DOI - PubMed
LinkOut - more resources
Full Text Sources

