Correlation Between Apparent Diffusion Coefficient and the Ki-67 Proliferation Index in Grading Pediatric Glioma
- PMID: 36957971
- PMCID: PMC10045956
- DOI: 10.1097/RCT.0000000000001400
Correlation Between Apparent Diffusion Coefficient and the Ki-67 Proliferation Index in Grading Pediatric Glioma
Abstract
Objective: This study aimed to investigate the correlation between apparent diffusion coefficient (ADC) and the Ki-67 proliferation index with the pathologic grades of pediatric glioma and to compare their diagnostic performance in differentiating grades of pediatric glioma.
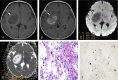
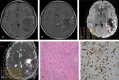
Patients and methods: Magnetic resonance imaging examinations and histopathologies of 121 surgically treated pediatric gliomas (87 low-grade gliomas [LGGs; grades 1 and 2] and 34 high-grade gliomas [HGGs; grades 3 and 4]) were retrospectively reviewed. The mean tumor ADC (ADCmean), minimum tumor ADC (ADCmin), tumor/normal brain ADC ratio (ADC ratio), and value of the Ki-67 proliferation index of LGGs and HGGs were compared. Correlation coefficients were calculated for ADC parameters and Ki-67 values. The receiver operating characteristic curve was used to determine the diagnostic value of ADCmean, ADCmin, ADC ratio, and Ki-67 proliferation index for differentiating LGGs and HGGs.
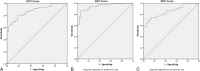
Results: The ADC values were significantly negatively correlated with glioma grade, and the Ki-67 proliferation index had a significant positive correlation with glioma grade. A significant negative correlation was observed between ADCmean and Ki-67 proliferation index, between ADCmin and Ki-67 proliferation index, and between ADC ratio and Ki-67 proliferation index. The receiver operating characteristic analysis demonstrated moderate to good accuracy for ADCmean in discriminating LGGs from HGGs (area under the curve [AUC], 0.875; sensitivity, 79.3%; specificity, 82.4%; accuracy, 80.2%; positive predictive value [PPV], 92.0%; and negative predictive value [NPV], 60.9% [cutoff value, 1.187] [×10-3 mm2/s]). Minimum tumor ADC showed very good to excellent accuracy with AUC of 0.946, sensitivity of 86.2%, specificity of 94.1%, accuracy of 88.4%, PPV of 97.4%, and NPV of 72.7% (cutoff value, 0.970) (×10-3 mm2/s). The ADC ratio showed moderate to good accuracy with AUC of 0.854, sensitivity of 72.4%, specificity of 88.2%, accuracy of 76.9%, PPV of 94.0%, and NPV of 55.6% (cutoff value, 1.426). For the parameter of the Ki-67 proliferation index, in discriminating LGGs from HGGs, very good to excellent diagnostic accuracy was observed (AUC, 0.962; sensitivity, 94.1%; specificity, 89.7%; accuracy, 90.9%; PPV, 97.5%; and NPV, 78.0% [cutoff value, 7]).
Conclusions: Apparent diffusion coefficient parameters and the Ki-67 proliferation index were significantly correlated with histological grade in pediatric gliomas. Apparent diffusion coefficient was closely correlated with the proliferative potential of pediatric gliomas. In addition, ADCmin showed superior performance compared with ADCmean and ADC ratio in differentiating pediatric glioma grade, with a close diagnostic efficacy to the Ki-67 proliferation index.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Diagnostic Value of Apparent Diffusion Coefficient and Proton Magnetic Resonance Spectroscopy in the Grading of Pediatric Gliomas.J Comput Assist Tomogr. 2021 Mar-Apr 01;45(2):269-276. doi: 10.1097/RCT.0000000000001130. J Comput Assist Tomogr. 2021. PMID: 33346568 Free PMC article.
-
The role of apparent diffusion coefficient in the grading of adult isocitrate dehydrogenase-mutant astrocytomas: relationship with the Ki-67 proliferation index.Acta Radiol. 2024 May;65(5):489-498. doi: 10.1177/02841851241242653. Epub 2024 Apr 22. Acta Radiol. 2024. PMID: 38644751
-
Diagnostic value of apparent diffusion coefficient in differentiating between high-grade gliomas and brain metastases.Acta Radiol. 2018 May;59(5):599-605. doi: 10.1177/0284185117727787. Epub 2017 Aug 23. Acta Radiol. 2018. PMID: 28835111
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article. Review.
-
The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: A systematic review and meta-analysis.Eur Radiol. 2016 Aug;26(8):2670-84. doi: 10.1007/s00330-015-4046-z. Epub 2015 Oct 15. Eur Radiol. 2016. PMID: 26471274 Review.
Cited by
-
Beyond invasive biopsies: using VASARI MRI features to predict grade and molecular parameters in gliomas.Cancer Imaging. 2024 Jan 2;24(1):3. doi: 10.1186/s40644-023-00638-8. Cancer Imaging. 2024. PMID: 38167551 Free PMC article.
-
Prognostic utility and characteristics of MIB-1 labeling index as a proliferative activity marker in childhood low-grade glioma: a retrospective observational study.J Cancer Res Clin Oncol. 2024 Apr 5;150(4):178. doi: 10.1007/s00432-024-05701-w. J Cancer Res Clin Oncol. 2024. PMID: 38580878 Free PMC article.
References
-
- Ryall S, Tabori U, Hawkins C. A comprehensive review of paediatric low-grade diffuse glioma: pathology, molecular genetics and treatment. Brain Tumor Pathol. 2017;34:51–61. - PubMed
-
- Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35:2370–2377. - PubMed
-
- Tian H Gou Y Pan Y, et al. . Quality appraisal of clinical practice guidelines on glioma. Neurosurg Rev. 2015;38:39–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

