MicroRNA156 conditions auxin sensitivity to enable growth plasticity in response to environmental changes in Arabidopsis
- PMID: 36949101
- PMCID: PMC10033679
- DOI: 10.1038/s41467-023-36774-9
MicroRNA156 conditions auxin sensitivity to enable growth plasticity in response to environmental changes in Arabidopsis
Abstract
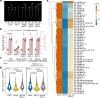
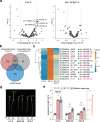
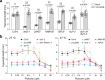
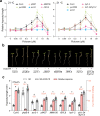
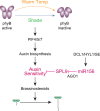
MicroRNAs (miRNAs) play diverse roles in plant development, but whether and how miRNAs participate in thermomorphogenesis remain ambiguous. Here we show that HYPONASTIC LEAVES 1 (HYL1)-a key component of miRNA biogenesis-acts downstream of the thermal regulator PHYTOCHROME INTERACTING FACTOR 4 in the temperature-dependent plasticity of hypocotyl growth in Arabidopsis. A hyl1-2 suppressor screen identified a dominant dicer-like1 allele that rescues hyl1-2's defects in miRNA biogenesis and thermoresponsive hypocotyl elongation. Genome-wide miRNA and transcriptome analysis revealed microRNA156 (miR156) and its target SQUAMOSA PROMOTER-BINDING-PROTEIN-LIKE 9 (SPL9) to be critical regulators of thermomorphogenesis. Surprisingly, perturbation of the miR156/SPL9 module disengages seedling responsiveness to warm temperatures by impeding auxin sensitivity. Moreover, miR156-dependent auxin sensitivity also operates in the shade avoidance response at lower temperatures. Thus, these results unveil the miR156/SPL9 module as a previously uncharacterized genetic circuit that enables plant growth plasticity in response to environmental temperature and light changes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
HYL1 controls the miR156-mediated juvenile phase of vegetative growth.J Exp Bot. 2012 Apr;63(7):2787-98. doi: 10.1093/jxb/err465. Epub 2012 Jan 20. J Exp Bot. 2012. PMID: 22268150 Free PMC article.
-
EIN3-Mediated Ethylene Signaling Attenuates Auxin Response during Hypocotyl Thermomorphogenesis.Plant Cell Physiol. 2021 Sep 24;62(4):708-720. doi: 10.1093/pcp/pcab028. Plant Cell Physiol. 2021. PMID: 33594435
-
Strontium stress disrupts miRNA biogenesis by reducing HYL1 protein levels in Arabidopsis.Ecotoxicol Environ Saf. 2020 Nov;204:111056. doi: 10.1016/j.ecoenv.2020.111056. Epub 2020 Aug 4. Ecotoxicol Environ Saf. 2020. PMID: 32763566
-
The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module regulates ambient temperature-responsive flowering via FLOWERING LOCUS T in Arabidopsis.Plant Physiol. 2012 May;159(1):461-78. doi: 10.1104/pp.111.192369. Epub 2012 Mar 16. Plant Physiol. 2012. PMID: 22427344 Free PMC article.
-
PIF7 is a master regulator of thermomorphogenesis in shade.Nat Commun. 2022 Aug 29;13(1):4942. doi: 10.1038/s41467-022-32585-6. Nat Commun. 2022. PMID: 36038577 Free PMC article.
Cited by
-
Temporal regulation of vegetative phase change in plants.Dev Cell. 2024 Jan 8;59(1):4-19. doi: 10.1016/j.devcel.2023.11.010. Dev Cell. 2024. PMID: 38194910 Free PMC article. Review.
-
Impact of Exogenous dsRNA on miRNA Composition in Arabidopsis thaliana.Plants (Basel). 2024 Aug 22;13(16):2335. doi: 10.3390/plants13162335. Plants (Basel). 2024. PMID: 39204771 Free PMC article.
-
Conserved plant transcriptional responses to microgravity from two consecutive spaceflight experiments.Front Plant Sci. 2024 Jan 8;14:1308713. doi: 10.3389/fpls.2023.1308713. eCollection 2023. Front Plant Sci. 2024. PMID: 38259952 Free PMC article.
-
Dysbindin Domain-Containing 1 in Prostate Cancer: New Insights into Bioinformatic Validation of Molecular and Immunological Features.Int J Mol Sci. 2023 Jul 25;24(15):11930. doi: 10.3390/ijms241511930. Int J Mol Sci. 2023. PMID: 37569304 Free PMC article.
-
Integrative phenotyping analyses reveal the relevance of the phyB-PIF4 pathway in Arabidopsis thaliana reproductive organs at high ambient temperature.BMC Plant Biol. 2024 Jul 29;24(1):721. doi: 10.1186/s12870-024-05394-w. BMC Plant Biol. 2024. PMID: 39075366 Free PMC article.

