Rational development of multicomponent mRNA vaccine candidates against mpox
- PMID: 36947428
- PMCID: PMC10071941
- DOI: 10.1080/22221751.2023.2192815
Rational development of multicomponent mRNA vaccine candidates against mpox
Abstract
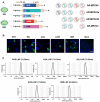
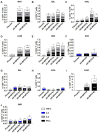
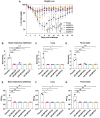
The re-emerging mpox (formerly monkeypox) virus (MPXV), a member of Orthopoxvirus genus together with variola virus (VARV) and vaccinia virus (VACV), has led to public health emergency of international concern since July 2022. Inspired by the unprecedent success of coronavirus disease 2019 (COVID-19) mRNA vaccines, the development of a safe and effective mRNA vaccine against MPXV is of high priority. Based on our established lipid nanoparticle (LNP)-encapsulated mRNA vaccine platform, we rationally constructed and prepared a panel of multicomponent MPXV vaccine candidates encoding different combinations of viral antigens including M1R, E8L, A29L, A35R, and B6R. In vitro and in vivo characterization demonstrated that two immunizations of all mRNA vaccine candidates elicit a robust antibody response as well as antigen-specific Th1-biased cellular response in mice. Importantly, the penta- and tetra-component vaccine candidates AR-MPXV5 and AR-MPXV4a showed superior capability of inducing neutralizing antibodies as well as of protecting from VACV challenge in mice. Our study provides critical insights to understand the protection mechanism of MPXV infection and direct evidence supporting further clinical development of these multicomponent mRNA vaccine candidates.
Keywords: Mpox virus; mRNA vaccine; mouse model; multicomponent; protective antigen.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Evaluation and comparison of immune responses induced by two Mpox mRNA vaccine candidates in mice.J Med Virol. 2023 Oct;95(10):e29140. doi: 10.1002/jmv.29140. J Med Virol. 2023. PMID: 37800627
-
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus.Signal Transduct Target Ther. 2023 Apr 28;8(1):172. doi: 10.1038/s41392-023-01432-5. Signal Transduct Target Ther. 2023. PMID: 37117161 Free PMC article.
-
Mpox virus mRNA-lipid nanoparticle vaccine candidates evoke antibody responses and drive protection against the Vaccinia virus challenge in mice.Antiviral Res. 2023 Aug;216:105668. doi: 10.1016/j.antiviral.2023.105668. Epub 2023 Jul 8. Antiviral Res. 2023. PMID: 37429529
-
Strategy of developing nucleic acid-based universal monkeypox vaccine candidates.Front Immunol. 2022 Oct 27;13:1050309. doi: 10.3389/fimmu.2022.1050309. eCollection 2022. Front Immunol. 2022. PMID: 36389680 Free PMC article. Review.
-
Current Status of Vaccine Development for Monkeypox Virus.Adv Exp Med Biol. 2024;1451:289-300. doi: 10.1007/978-3-031-57165-7_18. Adv Exp Med Biol. 2024. PMID: 38801585 Review.
Cited by
-
Rational design of a 'two-in-one' immunogen DAM drives potent immune response against mpox virus.Nat Immunol. 2024 Feb;25(2):307-315. doi: 10.1038/s41590-023-01715-7. Epub 2024 Jan 5. Nat Immunol. 2024. PMID: 38182667
-
A Quadrivalent mRNA Immunization Elicits Potent Immune Responses against Multiple Orthopoxviral Antigens and Neutralization of Monkeypox Virus in Rodent Models.Vaccines (Basel). 2024 Apr 5;12(4):385. doi: 10.3390/vaccines12040385. Vaccines (Basel). 2024. PMID: 38675767 Free PMC article.
-
Neutralization Determinants on Poxviruses.Viruses. 2023 Dec 8;15(12):2396. doi: 10.3390/v15122396. Viruses. 2023. PMID: 38140637 Free PMC article. Review.
-
Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity.Sci China Life Sci. 2023 Oct;66(10):2329-2341. doi: 10.1007/s11427-023-2378-x. Epub 2023 Jun 1. Sci China Life Sci. 2023. PMID: 37300753 Free PMC article.
-
Single-chain A35R-M1R-B6R trivalent mRNA vaccines protect mice against both mpox virus and vaccinia virus.EBioMedicine. 2024 Nov;109:105392. doi: 10.1016/j.ebiom.2024.105392. Epub 2024 Oct 18. EBioMedicine. 2024. PMID: 39423738 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
