Calcium signaling in astrocytes and gliotransmitter release
- PMID: 36937570
- PMCID: PMC10017551
- DOI: 10.3389/fnsyn.2023.1138577
Calcium signaling in astrocytes and gliotransmitter release
Abstract
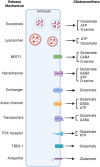
Glia are as numerous in the brain as neurons and widely known to serve supportive roles such as structural scaffolding, extracellular ionic and neurotransmitter homeostasis, and metabolic support. However, over the past two decades, several lines of evidence indicate that astrocytes, which are a type of glia, play active roles in neural information processing. Astrocytes, although not electrically active, can exhibit a form of excitability by dynamic changes in intracellular calcium levels. They sense synaptic activity and release neuroactive substances, named gliotransmitters, that modulate neuronal activity and synaptic transmission in several brain areas, thus impacting animal behavior. This "dialogue" between astrocytes and neurons is embodied in the concept of the tripartite synapse that includes astrocytes as integral elements of synaptic function. Here, we review the recent work and discuss how astrocytes via calcium-mediated excitability modulate synaptic information processing at various spatial and time scales.
Keywords: astrocyte; calcium signaling; gliotransmission; plasticity; tripartite synapse.
Copyright © 2023 Goenaga, Araque, Kofuji and Herrera Moro Chao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
G-Protein-Coupled Receptors in Astrocyte-Neuron Communication.Neuroscience. 2021 Feb 21;456:71-84. doi: 10.1016/j.neuroscience.2020.03.025. Epub 2020 Mar 26. Neuroscience. 2021. PMID: 32224231 Free PMC article. Review.
-
Gliotransmission and the tripartite synapse.Adv Exp Med Biol. 2012;970:307-31. doi: 10.1007/978-3-7091-0932-8_14. Adv Exp Med Biol. 2012. PMID: 22351062 Review.
-
Dysregulation of Astrocyte-Neuronal Communication in Alzheimer's Disease.Int J Mol Sci. 2021 Jul 23;22(15):7887. doi: 10.3390/ijms22157887. Int J Mol Sci. 2021. PMID: 34360652 Free PMC article. Review.
-
Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. PMID: 26269921 Free Books & Documents. Review.
-
Diversity and Specificity of Astrocyte-neuron Communication.Neuroscience. 2019 Jan 1;396:73-78. doi: 10.1016/j.neuroscience.2018.11.010. Epub 2018 Nov 17. Neuroscience. 2019. PMID: 30458223 Free PMC article. Review.
Cited by
-
Analog neuromorphic circuit for spontaneous Ca2+ oscillations.Sci Rep. 2023 Nov 16;13(1):20107. doi: 10.1038/s41598-023-47433-w. Sci Rep. 2023. PMID: 37973824 Free PMC article.
-
Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier.Cells. 2024 Jan 12;13(2):150. doi: 10.3390/cells13020150. Cells. 2024. PMID: 38247841 Free PMC article. Review.
-
Astrocyte-Mediated Neuroinflammation in Neurological Conditions.Biomolecules. 2024 Sep 25;14(10):1204. doi: 10.3390/biom14101204. Biomolecules. 2024. PMID: 39456137 Free PMC article. Review.
-
Selective stimulation of calcium signalling pathways in astrocytes with graphene electrodes.Nat Nanotechnol. 2024 Sep;19(9):1253-1254. doi: 10.1038/s41565-024-01712-3. Nat Nanotechnol. 2024. PMID: 39014183 No abstract available.
-
Investigating cocaine- and abstinence-induced effects on astrocyte gene expression in the nucleus accumbens.bioRxiv [Preprint]. 2024 Aug 5:2024.08.05.606656. doi: 10.1101/2024.08.05.606656. bioRxiv. 2024. PMID: 39149305 Free PMC article. Preprint.
References
-
- Abbracchio M., Verderio C. (2006). Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 276 91–103. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

