Adolescent binge ethanol impacts H3K36me3 regulation of synaptic genes
- PMID: 36937047
- PMCID: PMC10020663
- DOI: 10.3389/fnmol.2023.1082104
Adolescent binge ethanol impacts H3K36me3 regulation of synaptic genes
Abstract
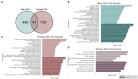
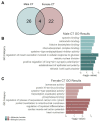
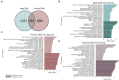
Adolescence is marked in part by the ongoing development of the prefrontal cortex (PFC). Binge ethanol use during this critical stage in neurodevelopment induces significant structural changes to the PFC, as well as cognitive and behavioral deficits that can last into adulthood. Previous studies showed that adolescent binge ethanol causes lasting deficits in working memory, decreases in the expression of chromatin remodeling genes responsible for the methylation of histone 3 lysine 36 (H3K36), and global decreases in H3K36 in the PFC. H3K36me3 is present within the coding region of actively-transcribed genes, and safeguards against aberrant, cryptic transcription by RNA Polymerase II. We hypothesize that altered methylation of H3K36 could play a role in adolescent binge ethanol-induced memory deficits. To investigate this at the molecular level, ethanol (4 g/kg, i.g.) or water was administered intermittently to adolescent mice. RNA-and ChIP-sequencing were then performed within the same tissue to determine gene expression changes and identify genes and loci where H3K36me3 was disrupted by ethanol. We further assessed ethanol-induced changes at the transcription level with differential exon-use and cryptic transcription analysis - a hallmark of decreased H3K36me3. Here, we found ethanol-induced changes to the gene expression and H3K36me3-regulation of synaptic-related genes in all our analyses. Notably, H3K36me3 was differentially trimethylated between ethanol and control conditions at synaptic-related genes, and Snap25 and Cplx1 showed evidence of cryptic transcription in males and females treated with ethanol during adolescence. Our results provide preliminary evidence that ethanol-induced changes to H3K36me3 during adolescent neurodevelopment may be linked to synaptic dysregulation at the transcriptional level, which may explain the reported ethanol-induced changes to PFC synaptic function.
Keywords: ChIP-seq; H3K36me3; PFC; RNA-seq; adolescent ethanol; alcohol; cryptic transcription; epigenetics.
Copyright © 2023 Brocato and Wolstenholme.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Adolescent binge ethanol impacts H3K9me3-occupancy at synaptic genes and the regulation of oligodendrocyte development.Front Mol Neurosci. 2024 May 22;17:1389100. doi: 10.3389/fnmol.2024.1389100. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38840776 Free PMC article.
-
Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC.Front Mol Neurosci. 2017 Sep 26;10:307. doi: 10.3389/fnmol.2017.00307. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29018328 Free PMC article.
-
Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current.J Neurosci. 2018 Jul 4;38(27):6207-6222. doi: 10.1523/JNEUROSCI.0550-18.2018. Epub 2018 Jun 18. J Neurosci. 2018. PMID: 29915134 Free PMC article.
-
Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence?Physiol Behav. 2015 Sep 1;148:122-30. doi: 10.1016/j.physbeh.2015.01.027. Epub 2015 Jan 23. Physiol Behav. 2015. PMID: 25624108 Free PMC article. Review.
-
H3K36 trimethylation-mediated biological functions in cancer.Clin Epigenetics. 2021 Oct 29;13(1):199. doi: 10.1186/s13148-021-01187-2. Clin Epigenetics. 2021. PMID: 34715919 Free PMC article. Review.
Cited by
-
Adolescent social housing protects against adult emotional and cognitive deficits and alters the PFC and NAc transcriptome in male and female C57BL/6J mice.Front Neurosci. 2023 Dec 7;17:1287584. doi: 10.3389/fnins.2023.1287584. eCollection 2023. Front Neurosci. 2023. PMID: 38130694 Free PMC article.
-
Adolescent binge ethanol impacts H3K9me3-occupancy at synaptic genes and the regulation of oligodendrocyte development.Front Mol Neurosci. 2024 May 22;17:1389100. doi: 10.3389/fnmol.2024.1389100. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38840776 Free PMC article.
-
Epigenetic regulation of microglia and neurons by proinflammatory signaling following adolescent intermittent ethanol (AIE) exposure and in human AUD.Adv Drug Alcohol Res. 2024 Mar 8;4:12094. doi: 10.3389/adar.2024.12094. eCollection 2024. Adv Drug Alcohol Res. 2024. PMID: 38524847 Free PMC article. Review.
References
-
- Acheson S. K., Bearison C., Risher M. L., Abdelwahab S. H., Wilson W. A., Swartzwelder H. S. (2013). Effects of acute or chronic ethanol exposure during adolescence on behavioral inhibition and efficiency in a modified water maze task. PLoS One 8, –e77768. doi: 10.1371/journal.pone.0077768, PMID: - DOI - PMC - PubMed
-
- Adkins A. E., Hack L. M., Bigdeli T. B., Williamson V. S., McMichael G. O., Mamdani M., et al. . (2017). Genomewide association study of alcohol dependence identifies risk loci altering ethanol-response behaviors in model organisms. Alcohol. Clin. Exp. Res. 41, 911–928. doi: 10.1111/acer.13362, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

