Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome
- PMID: 36936973
- PMCID: PMC10016348
- DOI: 10.3389/fimmu.2023.1086232
Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome
Abstract
Introduction: Polycystic Ovary Syndrome (PCOS) is the most common reproductive endocrine disorder among women of reproductive age, which is one of the main causes of anovulatory infertility. Even though the rapidly developed assisted reproductive technology (ART) could effectively solve fertility problems, some PCOS patients still have not obtained satisfactory clinical outcomes. The poor quality of oocytes caused by the abnormal follicular development of PCOS may directly contribute to the failure of ART treatment. Ovarian granulosa cells (GCs) are the most closely related cells to oocytes, and changes in their functional status have a direct impact on oocyte formation. Previous studies have shown that changes in the ovarian microenvironment, like oxidative stress and inflammation, may cause PCOS-related aberrant follicular development by impairing the physiological state of the GCs. Therefore, optimizing the ovarian microenvironment is a feasible method for enhancing the development potential of PCOS oocytes.
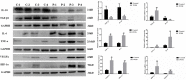
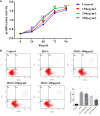
Methods: In this study, we first detected the expression of inflammatory-related factors (TGF-β1, IL-10, TNFα, IL-6) and oxidative stress-related factors (HIF-1α and VEGFA), as well as the proliferation ability and apoptosis level of GCs, which were collected from control patients (non-PCOS) and PCOS patients, respectively. Subsequently, human ovarian granulosa cell line (KGN) cells were used to verify the anti-inflammatory and anti-oxidative stress effects of chitosan oligosaccharide (COS) on GCs, as well as to investigate the optimal culture time and concentration of COS. The optimal culture conditions were then used to culture GCs from PCOS patients and control patients.
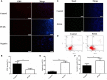
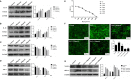
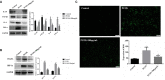
Results: The results showed that GCs from PCOS patients exhibited obvious inflammation and oxidative stress and significantly reduced proliferation and increased apoptosis. Furthermore, COS can increase the expression of anti-inflammatory factors (TGF-β1 and IL-10) and decrease the expression of pro-inflammatory factors (TNFα and IL-6), as well as promote the proliferation of GCs. Moreover, we found that COS can reduce the level of reactive oxygen species in GCs under oxidative stress by inhibiting the expression of HIF-1α and VEGFA and by suppressing the apoptosis of GCs induced by oxidative stress.
Conclusion: We find that inflammation and oxidative stress exist in the GCs of PCOS patients, and COS can reduce these factors, thereby improving the function of GCs.
Keywords: chitosan oligosaccharide; granulosa cells; inflammation; oxidative stress; polycystic ovary syndrome.
Copyright © 2023 Xie, Hong, Li, Ling, Zhou, Dai, Wu, Weng, Zhong, Tan and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment.Front Immunol. 2021 May 17;12:685724. doi: 10.3389/fimmu.2021.685724. eCollection 2021. Front Immunol. 2021. PMID: 34079559 Free PMC article.
-
Chitosan Oligosaccharides Alleviate H2O2-stimulated Granulosa Cell Damage via HIF-1α Signaling Pathway.Oxid Med Cell Longev. 2022 Apr 1;2022:4247042. doi: 10.1155/2022/4247042. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35401926 Free PMC article.
-
Expression and Clinical Significance of HIF-1α in Follicular Fluid and Granulosa Cells in Infertile PCOS Patients.Reprod Sci. 2023 Jul;30(7):2263-2274. doi: 10.1007/s43032-022-01135-2. Epub 2023 Jan 23. Reprod Sci. 2023. PMID: 36690916
-
A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS).J Assist Reprod Genet. 2022 Nov;39(11):2439-2473. doi: 10.1007/s10815-022-02625-7. Epub 2022 Oct 3. J Assist Reprod Genet. 2022. PMID: 36190593 Free PMC article. Review.
-
Disturbed Follicular Microenvironment in Polycystic Ovary Syndrome: Relationship to Oocyte Quality and Infertility.Endocrinology. 2024 Feb 20;165(4):bqae023. doi: 10.1210/endocr/bqae023. Endocrinology. 2024. PMID: 38375912 Review.
Cited by
-
Immunity and reproduction protective effects of Chitosan Oligosaccharides in Cyclophosphamide/Busulfan-induced premature ovarian failure model mice.Front Immunol. 2023 May 9;14:1185921. doi: 10.3389/fimmu.2023.1185921. eCollection 2023. Front Immunol. 2023. PMID: 37228612 Free PMC article.
-
Intra-ovarian inflammatory states and their associations with embryo quality in normal-BMI PCOS patients undergoing IVF treatment.Reprod Biol Endocrinol. 2024 Jan 11;22(1):11. doi: 10.1186/s12958-023-01183-6. Reprod Biol Endocrinol. 2024. PMID: 38212789 Free PMC article.
-
Identification and validation of novel genes related to immune microenvironment in polycystic ovary syndrome.Medicine (Baltimore). 2024 Oct 25;103(43):e40229. doi: 10.1097/MD.0000000000040229. Medicine (Baltimore). 2024. PMID: 39470566 Free PMC article.
-
Chitosan alleviates ovarian aging by enhancing macrophage phagocyte-mediated tissue homeostasis.Immun Ageing. 2024 Jan 27;21(1):10. doi: 10.1186/s12979-024-00412-9. Immun Ageing. 2024. PMID: 38279177 Free PMC article.
-
Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization.Int J Mol Sci. 2023 Jun 27;24(13):10707. doi: 10.3390/ijms241310707. Int J Mol Sci. 2023. PMID: 37445884 Free PMC article.
References
-
- Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab (2001) 86(3):1318–23. doi: 10.1210/jcem.86.3.7318 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

